Combination CXCR4 and PD-1 Blockade Enhances Intratumoral Dendritic Cell Activation and Immune Responses Against Hepatocellular Carcinoma
- PMID: 39514263
- PMCID: PMC11788650
- DOI: 10.1158/2326-6066.CIR-24-0324
Combination CXCR4 and PD-1 Blockade Enhances Intratumoral Dendritic Cell Activation and Immune Responses Against Hepatocellular Carcinoma
Abstract
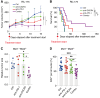
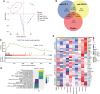
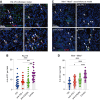
Immune checkpoint inhibitors have revolutionized the treatment of unresectable hepatocellular carcinoma (HCC), but their impressive efficacy is seen in just a fraction of patients. One key mechanism of immunotherapy resistance is the paucity of dendritic cells (DC) in liver malignancies. In this study, we tested combination blockade of PD-1 and CXCR4, a receptor for CXCL12, a pleiotropic factor that mediates immunosuppression in tumors. Using orthotopic grafted and autochthonous HCC models with underlying liver damage, we evaluated treatment feasibility and efficacy. In addition, we examined the effects of treatment using immunofluorescence, flow cytometric analysis of DCs in vivo and in vitro, and RNA sequencing. The combination anti-CXCR4 and anti-PD-1 therapy was safe and significantly inhibited tumor growth and prolonged survival in all murine preclinical models of HCC tested. The combination treatment successfully reprogrammed antigen-presenting cells, revealing the potential role of conventional type 1 DCs (cDC1) in the HCC microenvironment. Moreover, DC reprogramming enhanced anticancer immunity by facilitating CD8+ T-cell accumulation and activation in the HCC tissue. The effectiveness of anti-CXCR4/PD-1 therapy was compromised entirely in Batf3 knockout mice deficient in cDC1s. Thus, combined CXCR4/PD-1 blockade can reprogram intratumoral cDC1s and holds the potential to potentiate antitumor immune response against HCC.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S. Morita reports grants from Harvard Medical School, Massachusetts General Hospital, during the conduct of the study. K. Shigeta reports grants from The Uehara Memorial Foundation during the conduct of the study and grants from Japan Society of Laparoscopic Colorectal Surgery and Japan Society for the Promotion of Science outside the submitted work. T. Ando reports grants from Takeda Science Foundation during the conduct of the study. D.G. Duda reports grants from Bristol Myers Squibb during the conduct of the study and grants from Bayer, Surface Oncology, and Exelixis outside the submitted work. No disclosures were reported by the other authors.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–905. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

