Pharmacologically enabling the degradation of Na V 1.8 channels to reduce neuropathic pain
- PMID: 39514325
- PMCID: PMC12003070
- DOI: 10.1097/j.pain.0000000000003470
Pharmacologically enabling the degradation of Na V 1.8 channels to reduce neuropathic pain
Abstract
In phase II clinical trials, Na V 1.8 channels were identified as viable targets to treat acute pain. Results were modest, however, and Na V 1.8 pore blockers must be given systemically, potentially leading to adverse effects, especially during prolonged use. A local, long-lasting approach is desirable, yet local anesthetics are neither specific nor long-lasting. In lieu of a pore blocker approach, we show a pharmacological method targeting the scaffolding and degradation of Na V 1.8 channels, which attenuated neuropathic pain behavior in mice. Na V 1.8 channels interact with the WW domain-containing scaffold protein called Magi-1. WW domains are typically found in ubiquitin ligases, and Na V 1.8 channels are susceptible to degradation by ubiquitin ligases. Here, we show Na V 1.8 and MAGI-1 colocalized in human tissues. We demonstrate that a lipidated peptide derived from the Na V 1.8 WW binding domain, at sub-micromolar concentrations, inhibited rodent dorsal root ganglion neuronal firing. The peptide reduced Na V 1.8 channel immunoreactivity and tetrodotoxin-resistant currents in human dorsal root ganglion neurons. We found that the lipidated peptide attenuated neuropathic pain behaviors in mice for multiple weeks after a single injection. Our results reveal that the Na V 1.8-targeted lipidated peptide provides local and sustained analgesia, serving as a viable alternative to Na V 1.8 pore blockers.
Keywords: Dorsal root ganglion neurons; Electrophysiology; Neuropathic pain; Peptides; Scaffold protein; Sodium channels; Trafficking; Ubiquitination.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
A.B. is a cofounder of Channavix Therapeutics, LLC and Mimetic Medicines, Inc. A Patent Cooperation Treaty (PCT) application (serial number PCT/US2018/65,545) was filed on the use of lipidated peptidomimetics targeting NaV1.8 to induce local analgesia and to treat pain. Channavix Therapeutics has the exclusive license to the lipidated NaV1.8 peptidomimetic program from the Univesity at Buffalo. The remaining authors have no conflicts of interest to declare.
Data and materials availability: All data and materials used in this study will be made available upon reasonable request to the corresponding author.
Figures

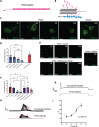
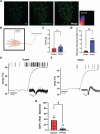
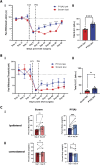
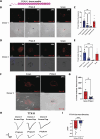
References
-
- Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, Freund-Mercier MJ, Barrot M. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain 2008;12:591–9. - PubMed
-
- Browne LE, Clare JJ, Wray D. Functional and pharmacological properties of human and rat NaV1.8 channels. Neuropharmacology 2009;56:905–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

