Distinctive physiology of molecularly identified medium spiny neurons in the macaque putamen
- PMID: 39514389
- PMCID: PMC11846625
- DOI: 10.1016/j.celrep.2024.114963
Distinctive physiology of molecularly identified medium spiny neurons in the macaque putamen
Abstract
The distinctive physiology of striatal medium spiny neurons (MSNs) underlies their ability to integrate sensory and motor input. In rodents, MSNs have a hyperpolarized resting potential and low input resistance. When activated, they have a delayed onset of spiking and regular spike rate. Here, we show that in the macaque putamen, latency to spike is reduced and spike rate adaptation is increased relative to mouse. We use whole-cell brain slice recordings and recover single-cell gene expression using Patch-seq to distinguish macaque MSN cell types. Species differences in the expression of ion channel genes including the calcium-activated chloride channel, ANO2, and an auxiliary subunit of the A-type potassium channel, DPP10, are correlated with species differences in spike rate adaptation and latency to the first spike, respectively. These surprising divergences in physiology better define the strengths and limitations of mouse models for understanding neuronal and circuit function in the primate basal ganglia.
Keywords: CP: Cell biology; CP: Neuroscience; Patch-Seq; cross-species divergence; latency to spike; macaque; medium spiny neurons; mouse; physiology; putamen; single-cell gene expression; spike rate adaptation; spike rates; striatum.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

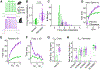


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

