Piezo1 Regulates Stiffness-Dependent DRG Axon Regeneration via Modifying Cytoskeletal Dynamics
- PMID: 39514408
- PMCID: PMC11653623
- DOI: 10.1002/advs.202405705
Piezo1 Regulates Stiffness-Dependent DRG Axon Regeneration via Modifying Cytoskeletal Dynamics
Abstract
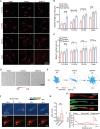
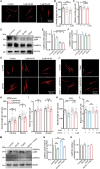
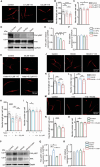
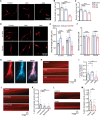
Despite medical interventions, the regenerative capacity of the peripheral nervous system is limited. Dorsal root ganglion (DRG) neurons possess the capacity to detect mechanical signals from their microenvironment, but the impact and mechanism by which these signals regulate axon regrowth and even regeneration in DRG neurons remain unclear. In this study, DRG neurons from newborn rats are cultured on substrates with varying degrees of stiffness in vitro to investigate the role of mechanical signals in axon regrowth. The findings reveal that substrate stiffness plays a crucial role in regulating axon regrowth, with an optimal stiffness required for this process. In addition, the data demonstrate that Piezo1, a mechanosensitive cation channel, detects substrate stiffness at the growth cone and regulates axon regrowth through activating downstream Ca2+-CaMKII-FAK-actin cascade signaling pathway. Interestingly, knocking down Piezo1 in adult rat DRG neurons leads to enhanced axon regeneration and accelerated recovery of sensory function after sciatic nerve injury. Overall, these findings contribute to the understanding of the role of mechanical signals in axon regeneration and highlight microenvironmental stiffness as a promising therapeutic target for repairing nerve injuries.
Keywords: DRG neurons; Piezo1; axon regeneration; substrate stiffness.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
