Inhibition of the miR-1914-5p increases the oxidative metabolism in cellular model of steatosis by modulating the Sirt1-PGC-1α pathway and systemic cellular activity
- PMID: 39514580
- PMCID: PMC11548759
- DOI: 10.1371/journal.pone.0313185
Inhibition of the miR-1914-5p increases the oxidative metabolism in cellular model of steatosis by modulating the Sirt1-PGC-1α pathway and systemic cellular activity
Abstract
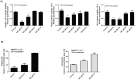
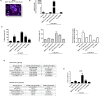
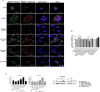
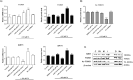
Metabolic associated fatty liver disease (MAFLD) is considered an indicator of metabolic syndrome, which affects millions of people around the world and no effective treatment is currently available. MAFLD involves a wide spectrum of liver damage, that initiates from steatosis (fatty live) and may progress to more complex pathophysiology. Then, details in lipid metabolism controlling should be explored aiming to control the fatty liver. In this context, the miR-1914-5p can be considered a potential biotechnology tool to control lipid metabolism in hepatic cells. This miRNA finds potential mRNA binding sequences in more than 100 molecules correlated with energy production and lipid metabolism pointed in bioinformatic platforms. The present study addressed the miR-1914-5p effects in hepatic HepG2/LX-2 co-cultured cells in a in vitro steatotic environment stablished by the addition of 400 μM of a mixture of oleic and palmitic acids. The analyses demonstrated that the inhibition of the miRNA reduced energetic metabolites such as total lipids, triglycerides, cholesterol and even glucose. In addition, the miR-inhibitor-transfected cells did not present any deleterious effect in cellular environment by controlling reactive oxygen species production (ROS), mitochondrial membrane potential (ΔΨm) and even the pro-inflammatory environment. Moreover, the functional effect of the investigated miR, suggested its close connection to the modulation of Sirt-1-PGC1-α pathway, a master switch metabolic route that controlls cellular energetic metabolism. Our assays also suggested a synergistic effect of this miR-1914-5p in cell metabolism, which should be considered as a strong candidate to control steatotic environment in future clinical trials.
Copyright: © 2024 Porto-Barbosa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Elucidation of SIRT-1/PGC-1α-associated mitochondrial dysfunction and autophagy in nonalcoholic fatty liver disease.Lipids Health Dis. 2021 Apr 26;20(1):40. doi: 10.1186/s12944-021-01461-5. Lipids Health Dis. 2021. PMID: 33902605 Free PMC article.
-
Artemisia capillaris Thunb. Water extract alleviates metabolic dysfunction-associated Steatotic liver disease Disease by inhibiting miR-34a-5p to activate Sirt1-mediated hepatic lipid metabolism.J Ethnopharmacol. 2025 Feb 10;338(Pt 2):119030. doi: 10.1016/j.jep.2024.119030. Epub 2024 Nov 6. J Ethnopharmacol. 2025. PMID: 39515682
-
Resistance Exercise Improves Glycolipid Metabolism and Mitochondrial Biogenesis in Skeletal Muscle of T2DM Mice via miR-30d-5p/SIRT1/PGC-1α Axis.Int J Mol Sci. 2024 Nov 19;25(22):12416. doi: 10.3390/ijms252212416. Int J Mol Sci. 2024. PMID: 39596482 Free PMC article.
-
miR-34a regulates lipid metabolism by targeting SIRT1 in non-alcoholic fatty liver disease with iron overload.Arch Biochem Biophys. 2020 Nov 30;695:108642. doi: 10.1016/j.abb.2020.108642. Epub 2020 Oct 21. Arch Biochem Biophys. 2020. PMID: 33098868
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
References
-
- Lambertucci F, Motiño O, Pérez-Lanzón M, Sijing LiS, Plantureux C, Pol J, et al.. Isolation of primary mouse hepatocytes and non-parenchymal cells from a liver with precancerous lesions. In: Kroemer G, Pol J, Martins I (eds) Liver Carcinogenesis. Springer US, New York, NY, 2024; pp 109–128. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

