Distinct septo-hippocampal cholinergic projections separately mediate stress-induced emotional and cognitive deficits
- PMID: 39514666
- PMCID: PMC11546849
- DOI: 10.1126/sciadv.ado1508
Distinct septo-hippocampal cholinergic projections separately mediate stress-induced emotional and cognitive deficits
Abstract
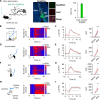
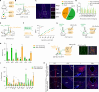
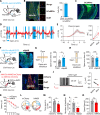
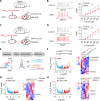
Patients suffering from chronic stress develop numerous symptoms, including emotional and cognitive deficits. The precise circuit mechanisms underlying different symptoms remain poorly understood. We identified two distinct basal forebrain cholinergic subpopulations in mice projecting to the dorsal hippocampus (dHPC) or ventral hippocampus (vHPC), which exhibited distinct input organizations, electrophysiological characteristics, transcriptomics, and responses to positive and negative valences of stimuli and were critical for cognitive and emotional modulation, respectively. Moreover, chronic stress induced elevated anxiety levels and cognitive deficits in mice, accompanied by enhanced vHPC but suppressed dHPC cholinergic projections. Chemogenetic activation of dHPC or inhibition of vHPC cholinergic projections alleviated stress-induced aberrant behaviors. Furthermore, we identified that the acetylcholinesterase inhibitor donepezil combined with blockade of muscarinic receptor 1-type muscarinic acetylcholine receptors in the vHPC rescued both stress-induced phenotypes. These data illuminated distinct septo-hippocampal cholinergic circuits mediated specific symptoms independently under stress, which may provide promising strategies for circuit-based treating of stress-related psychiatric disorders.
Figures



References
-
- Schwabe L., Hermans E. J., Joëls M., Roozendaal B., Mechanisms of memory under stress. Neuron 110, 1450–1467 (2022). - PubMed
-
- de Quervain D., Schwabe L., Roozendaal B., Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat. Rev. Neurosci. 18, 7–19 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

