Increased exercise tolerance in humanized G6PD-deficient mice
- PMID: 39514761
- PMCID: PMC11786683
- DOI: 10.1182/bloodadvances.2024013968
Increased exercise tolerance in humanized G6PD-deficient mice
Abstract
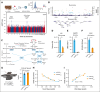
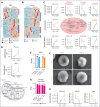
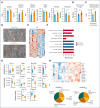
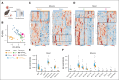
Glucose-6-phosphate dehydrogenase (G6PD) deficiency affects 500 million people globally, affecting red blood cell (RBC) antioxidant pathways and increasing susceptibility to hemolysis under oxidative stress. Despite the systemic generation of reactive oxygen species during exercise, the effects of exercise on individuals with G6PD deficiency remain poorly understood This study used humanized mouse models expressing the G6PD Mediterranean variant (S188F, with 10% enzymatic activity) to investigate exercise performance and molecular outcomes. Surprisingly, despite decreased enzyme activity, G6PD-deficient mice have faster critical speed than mice expressing human canonical G6PD. After exercise, deficient mice did not exhibit differences in RBC morphology or hemolysis, but had improved cardiac function, including cardiac output, stroke volume, sarcomere length, and mitochondrial content. Proteomics analyses of cardiac and skeletal muscles (gastrocnemius, soleus) from G6PD-deficient compared with sufficient mice revealed improvements in mitochondrial function and increased protein turnover via ubiquitination, especially for mitochondrial and structural myofibrillar proteins. Mass spectrometry-based metabolomics revealed alterations in energy metabolism and fatty acid oxidation. These findings challenge the traditional assumptions regarding hemolytic risk during exercise in G6PD deficiency, suggesting a potential metabolic advantage in exercise performance for individuals carrying noncanonical G6PD variants.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A. D'Alessandro, K.C.H., and T.N. are founders of Omix Technologies Inc and Altis Biosciences LLC. A. D'Alessandro is a scientific advisory board member for Hemanext Inc and Macopharma Inc. The remaining authors declare no competing financial interests.
Figures



References
-
- Luzzatto L, Ally M, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Blood. 2020;136(11):1225–1240. - PubMed
-
- Lee J, Poitras BT. Prevalence of glucose-6-phosphate dehydrogenase deficiency, U.S. Armed Forces, May 2004-September 2018. MSMR. 2019;26(12):14–17. - PubMed
-
- Nemkov T, Reisz JA, Xia Y, Zimring JC, D'Alessandro A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev Proteomics. 2018;15(11):855–864. - PubMed

