Exploiting blood-based biomarkers to align preclinical models with human traumatic brain injury
- PMID: 39514789
- PMCID: PMC11967814
- DOI: 10.1093/brain/awae350
Exploiting blood-based biomarkers to align preclinical models with human traumatic brain injury
Abstract
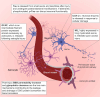
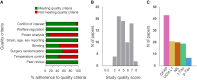
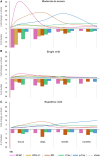
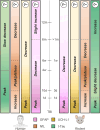
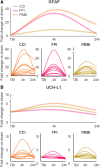
Rodent models are important research tools for studying the pathophysiology of traumatic brain injury (TBI) and developing new therapeutic interventions for this devastating neurological disorder. However, the failure rate for the translation of drugs from animal testing to human treatments for TBI is 100%. While there are several potential explanations for this, previous clinical trials have relied on extrapolation from preclinical studies for critical design considerations, including drug dose optimization, post-injury drug treatment initiation and duration. Incorporating clinically relevant biomarkers in preclinical studies may provide an opportunity to calibrate preclinical models to identical (or similar) measurements in humans, link to human TBI biomechanics and pathophysiology, and guide therapeutic decisions. To support this translational goal, we conducted a systematic literature review of preclinical TBI studies in rodents measuring blood levels of clinically used GFAP, UCH-L1, NfL, total-Tau (t-Tau) or phosphorylated-Tau (p-Tau) published in PubMed/EMBASE up to 10 April 2024. Although many factors influence clinical TBI outcomes, many of those cannot routinely be assessed in rodent studies (e.g. intracranial pressure monitoring). Thus we focused on blood biomarkers' temporal trajectories and discuss our findings in the context of the latest clinical TBI biomarker data. Of 805 original preclinical studies, 74 met the inclusion criteria, with a median quality score of 5 (25th-75th percentiles: 4-7) on the CAMARADES checklist. GFAP was measured in 43 studies, UCH-L1 in 21, NfL in 20, t-Tau in 19 and p-Tau in seven. Data from rodent models indicate that all biomarkers exhibited injury severity-dependent elevations with distinct temporal profiles. GFAP and UCH-L1 peaked within the first day after TBI (30- and 4-fold increases, respectively, in moderate-to-severe TBI versus sham), with the highest levels observed in the contusion TBI model. NfL peaked within days (18-fold increase) and remained elevated up to 6 months post-injury. GFAP and NfL show a pharmacodynamic response in 64.7% and 60%, respectively, of studies evaluating neuroprotective therapies in preclinical models. However, GFAP's rapid decline post-injury may limit its utility for understanding the response to new therapeutics beyond the hyperacute phase after experimental TBI. Furthermore, as in humans, subacute NfL levels inform on chronic white matter loss after TBI. t-Tau and p-Tau levels increased over weeks after TBI (up to 6- and 16-fold, respectively); however, their relationship with underlying neurodegeneration has yet to be addressed. Further investigation into biomarker levels in the subacute and chronic phases after TBI will be needed to fully understand the pathomechanisms underpinning blood biomarkers' trajectories and select the most suitable experimental model to optimally relate preclinical mechanistic studies to clinical observations in humans. This new approach could accelerate the translation of neuroprotective treatments from laboratory experiments to real-world clinical practices.
Keywords: blood biomarkers; disease trajectories; model calibration; translational research; traumatic brain injury.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
N.M. is scientific advisor of Teqcool Inc. and has received speaker honoraria for Balt International Inc.—of no relevance to the present study. V.D.P. declares conflict of interest with TBI biomarkers (sncRNAs) not mentioned in the present paper. K.K.W.W. is a share-holder of Gryphon Bio, Inc. and Owl Therapeutics, and receives patent inventor royalties from the University of Florida.
Figures
References
-
- Majdan M, Plancikova D, Brazinova A, et al. Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis. Lancet Public Health. 2016;1:e76–e83. - PubMed
-
- Kochanek PM, Bramlett HM, Dixon CE, et al. Approach to modeling, therapy evaluation, drug selection, and biomarker assessments for a multicenter Pre-clinical drug screening consortium for acute therapies in severe traumatic brain injury: Operation brain trauma therapy. J Neurotrauma. 2016;33:513–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

