ReNeu: A Pivotal, Phase IIb Trial of Mirdametinib in Adults and Children With Symptomatic Neurofibromatosis Type 1-Associated Plexiform Neurofibroma
- PMID: 39514826
- PMCID: PMC11825507
- DOI: 10.1200/JCO.24.01034
ReNeu: A Pivotal, Phase IIb Trial of Mirdametinib in Adults and Children With Symptomatic Neurofibromatosis Type 1-Associated Plexiform Neurofibroma
Erratum in
-
Erratum: ReNeu: A Pivotal, Phase IIb Trial of Mirdametinib in Adults and Children With Symptomatic Neurofibromatosis Type 1-Associated Plexiform Neurofibroma.J Clin Oncol. 2025 Jan 10;43(2):239. doi: 10.1200/JCO-24-02561. Epub 2024 Dec 2. J Clin Oncol. 2025. PMID: 39621958 Free PMC article. No abstract available.
Abstract
Purpose: Pharmacologic therapies for neurofibromatosis type 1-associated plexiform neurofibromas (NF1-PNs) are limited; currently, none are US Food and Drug Administration-approved for adults.
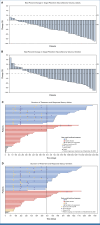
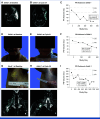
Methods: ReNeu is an open-label, multicenter, pivotal, phase IIb trial of mirdametinib in 58 adults (≥18 years of age) and 56 children (2 to 17 years of age) with NF1-PN causing significant morbidities. Patients received mirdametinib capsules or tablets for oral suspension (2 mg/m2 twice daily, maximum 4 mg twice daily), regardless of food intake, in 3 weeks on/1 week off 28-day cycles. The primary end point was confirmed objective response rate (ORR; proportion of patients with a ≥20% reduction of target PN volume from baseline on consecutive scans during the 24-cycle treatment phase) assessed by blinded independent central review (BICR) of volumetric magnetic resonance imaging.
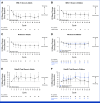
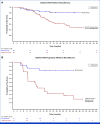
Results: Twenty-four of 58 adults (41%) and 29 of 56 children (52%) had a BICR-confirmed objective response during the 24-cycle treatment phase; in addition, two adults and one child had confirmed responses during long-term follow-up. Median (range) target PN volumetric best response was -41% (-90 to 13) in adults and -42% (-91 to 48) in children. Both cohorts reported significant and clinically meaningful improvement in patient- or parent proxy-reported outcome measures of worst tumor pain severity, pain interference, and health-related quality of life (HRQOL) that began early and were sustained during treatment. The most commonly reported treatment-related adverse events were dermatitis acneiform, diarrhea, and nausea in adults and dermatitis acneiform, diarrhea, and paronychia in children.
Conclusion: In ReNeu, the largest multicenter NF1-PN trial reported to date, mirdametinib treatment demonstrated significant confirmed ORRs by BICR, deep and durable PN volume reductions, and early, sustained, and clinically meaningful improvement in pain and HRQOL. Mirdametinib was well-tolerated in adults and children.
Trial registration: ClinicalTrials.gov NCT03962543.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Side L, Taylor B, Cayouette M, et al. Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. N Engl J Med. 1997;336:1713–1720. - PubMed
-
- Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1–11. - PubMed
-
- Prada CE, Rangwala FA, Martin LJ, et al. Pediatric plexiform neurofibromas: Impact on morbidity and mortality in neurofibromatosis type 1. J Pediatr. 2012;160:461–467. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

