Interleukin-1β induces trained innate immunity in human hematopoietic progenitor cells in vitro
- PMID: 39515317
- PMCID: PMC11751800
- DOI: 10.1016/j.stemcr.2024.09.004
Interleukin-1β induces trained innate immunity in human hematopoietic progenitor cells in vitro
Abstract
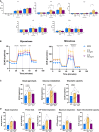
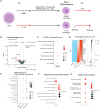
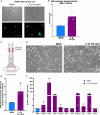
Innate immune cells can develop a long-lasting hyperresponsive phenotype, termed trained immunity, mediated by epigenetic and metabolic reprogramming. In mice, exposure to Bacille Calmette-Guérin (BCG), β-glucan, or Western diet induces trained immunity by reprogramming hematopoietic progenitor cells (HPCs), through interleukin-1β (IL-1β) signaling in the bone marrow (BM). We investigated whether IL-1β induces trained immunity in primary human BM-derived HPCs in vitro. We exposed human BM-derived HPCs to IL-1β for 4 h. HPCs were expanded and differentiated into monocytes followed by functional and transcriptomic characterization. IL-1β-exposed HPCs showed higher granulocyte-macrophage colony-forming units. The monocyte offspring produced more tumor necrosis factor (TNF) and IL-1β after restimulation with lipopolysaccharide (LPS) and Pam3Cys and is metabolically more active. Transcriptomic analysis showed upregulation of key atherogenic and inflammatory pathways. In conclusion, brief exposure of human BM-derived HPCs to IL-1β in vitro induces a trained immunity phenotype.
Keywords: IL-1β; bone marrow; hematopoietic progenitor cells; macrophages; monocytes; trained immunity.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.G.N. and L.A.B.J. are scientific founders of TTxD and Lemba TX. M.G.N. is scientific founder of Biotrip. W.H.C.R. is a consultant for Stryker for educational purposes only.
Figures


References
-
- Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010. - DOI - PubMed
-
- Bannister S., Kim B., Domínguez-Andrés J., Kilic G., Ansell B.R.E., Neeland M.R., Moorlag S.J.C.F.M., Matzaraki V., Vlahos A., Shepherd R., et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abn4002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

