STK33 as the functional substrate of miR-454-3p for suppression and apoptosis in neuroblastoma
- PMID: 39515612
- PMCID: PMC11863495
- DOI: 10.1016/j.mocell.2024.100145
STK33 as the functional substrate of miR-454-3p for suppression and apoptosis in neuroblastoma
Abstract
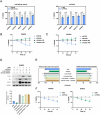
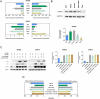
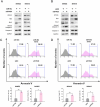
miR-454-3p has been reported to be a tumor-suppressive microRNA (miRNA) in multiple cancer types. We identified the kinase STK33 mRNA, which is a high-risk factor for survival in neuroblastoma (NB) patients, as being a substrate of miR-454-3p in NB. Even though STK33 is an attractive target for several cancers, the development of inhibitors of STK33 has been challenging. For the various cell lines tested, we demonstrated reduced growth and viability with the miR-454-3p mimic. From among the candidate NB-associated miRNAs, miR-454-3p mimic and its antagonist had the most profound effect on STK33 mRNA and protein-level changes. Under various conditions of growth and external stress for the cells, the RNA levels for miR-454-3p and STK33 also negatively correlated. Luciferase reporter assays demonstrated STK33 as a substrate for miR-454-3p, and recombinant versions of STK33 resistant to miR-454-3p significantly blunted the suppressive effect of the miR-454-3p and established STK33 as the major functional substrate of miR-454-3p. Overexpression of miR-454-3p or knockdown of STK33 mRNA promoted autophagy and at the same time, increased the apoptotic markers in the tested NB cells, indicating a mechanism for the suppressive effect of the agents. Given the difficult-to-drug targets such as STK33 and the recent successes in RNA delivery methods for cancer treatment, it is thought that targeting cancer cells with a suppressive miRNA such as miR-454-3p for STK33-dependent cancer types may be an alternative means of NB therapy.
Keywords: MiR-454-3p; Neuroblastoma; RNA targeting; Serine/threonine kinase 33; Suppressive microRNA.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF COMPETING INTERESTS There are no competing interests to declare.
Figures



Similar articles
-
LncRNA SNHG1 contributes to tumorigenesis and mechanism by targeting miR-338-3p to regulate PLK4 in human neuroblastoma.Eur Rev Med Pharmacol Sci. 2019 Oct;23(20):8971-8983. doi: 10.26355/eurrev_201910_19296. Eur Rev Med Pharmacol Sci. 2019. PMID: 31696485
-
microRNA-144-3p suppresses human neuroblastoma cell proliferation by targeting HOXA7.Eur Rev Med Pharmacol Sci. 2019 Jan;23(2):716-723. doi: 10.26355/eurrev_201901_16885. Eur Rev Med Pharmacol Sci. 2019. PMID: 30720179
-
LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p.Biomed Pharmacother. 2020 Sep;129:110268. doi: 10.1016/j.biopha.2020.110268. Epub 2020 Jun 17. Biomed Pharmacother. 2020. PMID: 32563146
-
MicroRNA-145 overexpression inhibits neuroblastoma tumorigenesis in vitro and in vivo.Bioengineered. 2020 Dec;11(1):219-228. doi: 10.1080/21655979.2020.1729928. Bioengineered. 2020. PMID: 32083506 Free PMC article.
-
Screen for Inhibitors of STK33 Kinase Activity.2011 Dec 16 [updated 2014 May 13]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2011 Dec 16 [updated 2014 May 13]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23658944 Free Books & Documents. Review.
References
-
- Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

