Prognostic impact of circulating tumor DNA detection in portal and peripheral blood in resected pancreatic ductal adenocarcinoma patients
- PMID: 39516243
- PMCID: PMC11549393
- DOI: 10.1038/s41598-024-76903-y
Prognostic impact of circulating tumor DNA detection in portal and peripheral blood in resected pancreatic ductal adenocarcinoma patients
Abstract
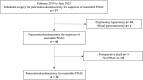
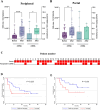
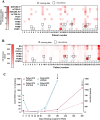
In PDAC patients, ctDNA detection's prognostic significance needs validation especially in resected patients. This study investigated ctDNA kinetics in portal and peripheral blood before and after resection, and whether tissue mobilization during surgery influences ctDNA detection. In this single-center prospective cohort, portal and peripheral blood were drawn during pancreaticoduodenectomy before and after tissue mobilization, during 12 postoperative months and were associated with overall survival (OS), recurrence-free survival (RFS) and CA19-9 (secondary endpoints). Tumor mutations were identified using next-generation-sequencing and ctDNA detected by digital droplet PCR. From 2018 to 2022, 34 patients were included. The 2-year RFS and OS were 47.6%(95%CI[29.5; 63.6]) and 65.7%(95%CI[46.5; 79.4]) respectively. Intraoperatively, ctDNA detection in portal or peripheral blood was associated with worse RFS (HR[95%CI]3.26[1.26; 8.45],p = 0.010) and OS (HR[95%CI]5.46[1.65;18.01],p = 0.002). Portal vein sampling did not improve ctDNA detection. CtDNA levels were increased by 2.5-fold (p = 0.031) in peripheral blood after tissue mobilization but not significantly linked to RFS or OS. Detecting ctDNA intraoperatively was correlated with poorer RFS (HR [95% CI] 3.26 [1.26;8.45], p = 0.010) and 0S (HR [95% CI] 5.46 [1.65;18.01], p = 0.002). Portal vein sampling did not improve ctDNA detection. Tissue mobilization increases ctDNA levels. Intraoperative detection of ctDNA is associated with a worse prognosis.
Keywords: Circulating tumor DNA; Liquid biopsy; Overall survival; Pancreatic ductal adenocarcinoma; Recurrence-free survival; Tissue mobilization.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA. Cancer J. Clin. 73, 17–48 (2023). - PubMed
-
- Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014). - PubMed
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 70, 7–30 (2020). - PubMed
-
- Conroy, T. et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 379, 2395–2406 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
- RC31/17/0327/Toulouse University Hospital
LinkOut - more resources
Full Text Sources
Medical

