Phase 1b/2 study of the liposomal formulation of eribulin (E7389-LF) in combination with nivolumab: Results from the phase 2 esophageal cancer cohort
- PMID: 39516370
- PMCID: PMC11523933
- DOI: 10.1038/s44276-024-00066-6
Phase 1b/2 study of the liposomal formulation of eribulin (E7389-LF) in combination with nivolumab: Results from the phase 2 esophageal cancer cohort
Abstract
Background: Esophageal cancer is one of the most common types of cancer in Japan. Herein, we report the efficacy and safety of E7389-LF plus the immune checkpoint inhibitor, nivolumab, from the esophageal cancer cohort of the phase 2 part of Study 120.
Methods: Eligible patients received E7389-LF 2.1 mg/m2 plus nivolumab 360 mg intravenously Q3W. The primary objective was to evaluate the objective response rate (ORR); other objectives included safety, progression-free survival (PFS), and overall survival (OS).
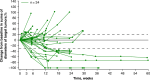
Results: Of the 35 Japanese patients enrolled, 7 (20.0%) had a partial response as their best overall response, and 14 (40.0%) had stable disease. The ORR was 20.0% (95% CI 8.4-36.9). The duration of response was 5.6 months (95% CI 1.7-not estimable [NE]). The median PFS was 2.81 months (95% CI 1.31-4.17). The median OS was not reached (95% CI 6.54 months-NE). The most common treatment-emergent adverse events were neutropenia (65.7%), pyrexia (60.0%), and leukopenia (57.1%). Select plasma endothelial cell markers levels increased from day 1 of cycle 1 and changes were pronounced between days 8-15 of each cycle.
Conclusions: E7389-LF plus nivolumab showed antitumor activity in patients with unresectable and pretreated esophageal cancer and should be evaluated further in a broader population.
Clinical trial registration: NCT04078295.
© 2024. The Author(s).
Conflict of interest statement
Akira Ooki: Honoraria - Bristol Myers Squibb Foundation, Daiichi Sankyo, Merck Serono, Ono Pharmaceutical. Sachiko Yamamoto: No Relationships to Disclose. Hisato Kawakami: Honoraria - Bayer, Bristol Myers Squibb Japan, Chugai/Roche, Daiichi Sankyo, Lilly Japan, Merck Serono, MSD K.K, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda, Yakult Pharmaceutical; Consulting or Advisory Role - Bristol Myers Squibb Japan, Daiichi Sankyo Co. Ltd., Lilly Japan, Ono Pharmaceutical, Taiho; Pharmaceutical Research Funding - Chugai Pharma (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Kobayashi Pharmaceutical (Inst). Tomoki Makino: No Relationships to Disclose. Akihito Kawazoe: Honoraria - Bristol Myers Squibb, Daiichi Sankyo, Lilly, Merck Serono, Ono Pharmaceutical, Taiho Pharmaceutical; Consulting or Advisory Role - MSD; Speakers' Bureau - Bristol Myers Squibb, Daiichi Sankyo, Lilly, Merck Serono, Ono Pharmaceutical, Taiho Pharmaceutical; Research Funding - AstraZeneca (Inst), Bayer (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Sumitomo Dainippon Pharma (Inst), Taiho Pharmaceutical (Inst) Toshiki Masuishi: Honoraria - Bayer Yakuhin, Bristol Myers Squibb, Chugai Pharma, Daiichi Sankyo, Lilly, Merck Serono, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, Takeda, Yakult Honsha; Research Funding - Amgen (Inst), Boehringer Ingelheim (Inst), CMIC (Inst), Daiichi Sankyo (Inst), Lilly Japan (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Syneos Health (Inst). Takahiro Tsushima: Honoraria - Lilly Japan, MSD; Ono Pharmaceutical, Taiho Pharmaceutical, Bristol Myers Squibb. Motohiro Hirao: No Relationships to Disclose. Naoki Takegawa: No Relationships to Disclose. Kaori Hino: No Relationships to Disclose. Satoru Iwasa: Honoraria – Bristol Myers Squibb Japan, Chugai Pharma, Daiichi Sankyo, Lilly Japan, Ono Pharmaceutical, Taiho Pharmaceutical; Research Funding - Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Merck Serono (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Seagen (Inst), Taiho Pharmaceutical (Inst), Zymeworks (Inst). Hiroki Hara: Honoraria - Asahi Kasei, Asahi Kasei, Bayer; Bristol Myers Squibb, Chugai Pharma, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly, Merck Serono, MSD, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, Takeda, Yakult Honsha; Consulting or Advisory Role - Boehringer Ingelheim, Bristol Myers Squibb Japan, Daiichi Sankyo/UCB Japan, Dainippon Sumitomo, MSD, Ono Pharmaceutical; Research Funding - ALX Oncology (Inst), Amgen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BeiGene (Inst), Boehringer Ingelheim (Inst), Chugai Pharma (Inst), Daiichi Sankyo (Inst), Dainippon Sumitomo Pharma (Inst), Janssen Oncology (Inst), Merck Serono (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst). Shota Kaname: Employment - Ono PharmaceuticalDaiko Matsuoka: Employment - Eisai. Yohei Otake: Employment - Eisai. Keisuke Yasuda: Employment - Eisai. Takao Takase: Employment - Eisai. Shuya Takashima: Employment - Eisai. Taro Semba: Employment - Eisai; Patents, Royalties, Other Intellectual Property - Eisai. Takashi Oshima: No Relationships to Disclose.
Figures


References
-
- The Global Cancer Observatory. Population fact sheets: Japan. Globocan 2020, https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-she... (accessed 28 September 2023).
-
- Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–58.e2. - PubMed
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Esophageal and Esophagogastric Junction Cancers. Version 3.2023, https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (accessed 28 September 2023).
-
- Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–62. - PubMed
-
- Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–17. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
