Survival benefit of cytoreductive surgery in patients with primary stage IV endometrial cancer: a systematic review & meta-analysis
- PMID: 39516418
- PMCID: PMC11523991
- DOI: 10.1038/s44276-024-00084-4
Survival benefit of cytoreductive surgery in patients with primary stage IV endometrial cancer: a systematic review & meta-analysis
Abstract
Background: This systematic review and meta-analysis aimed to investigate the survival outcomes following cytoreductive surgery (CRS) in patients with primary stage IV endometrial cancer (EC).
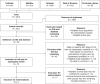
Methods: We systematically searched the Cochrane Library, Embase, MEDLINE/PubMed, and Web of Science for original studies reporting survival outcomes of primary stage IV EC after complete, optimal, and incomplete CRS. Pooled hazard ratios (HRs) for overall survival (OS) comparing optimal CRS with incomplete CRS were calculated using a random-effects model. Heterogeneity was assessed using the I2 and the Q-test.
Results: Twelve studies, including 748 patients, were analysed. 187 patients underwent complete CRS, and 146 patients optimal CRS. Ten studies reported a significant OS benefit after complete (18-48 months) and optimal CRS (13-34 months) compared to incomplete CRS (7-19 months). A benefit was also observed in patients with serous EC or extra- abdominal metastasis. Meta-analysis showed improved OS after complete/optimal vs. incomplete CRS (HR = 0.38, 95% CI 0.21-0.69, p = 0.0016). Heterogeneity was substantial between studies (I2 = 76.7%, p < 0.0001).
Conclusion: Our study supports considering CRS in all patients with primary stage IV EC if complete resection is deemed feasible, while also emphasizing the importance of weighing the harms and benefits of this extensive treatment and adopting shared decision-making.
Prospero registration: CRD42022302968 on May 10th, 2022.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL. et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S105–43. 10.1016/S0020-7292(06)60031-3. - PubMed
-
- Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novak Z, Black D, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023. 10.1056/NEJMoa2216334. - PubMed
-
- Abu-Rustum N, Yashar C, Arend R, Barber E, Bradley K, Brooks R, et al. Uterine neoplasms, version 2.2024, March 6, 2024; page 14, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2024. - PubMed
-
- Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39. 10.1136/ijgc-2020-002230. - PubMed
LinkOut - more resources
Full Text Sources
