Novel tools to quantify total, phospho-Ser129 and aggregated alpha-synuclein in the mouse brain
- PMID: 39516469
- PMCID: PMC11549080
- DOI: 10.1038/s41531-024-00830-y
Novel tools to quantify total, phospho-Ser129 and aggregated alpha-synuclein in the mouse brain
Abstract
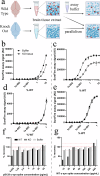
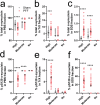
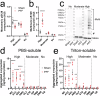
Assays for quantifying aggregated and phosphorylated (S129) human α-synuclein protein are widely used to evaluate pathological burden in patients suffering from synucleinopathy disorders. Many of these assays, however, do not cross-react with mouse α-synuclein or exhibit poor sensitivity for this target, which is problematic considering the preponderance of mouse models at the forefront of pre-clinical α-synuclein research. In this project, we addressed this unmet need by reformulating two existing AlphaLISA® SureFire® Ultra™ total and pS129 α-synuclein assay kits to yield robust and ultrasensitive (LLoQ ≤ 0.5 pg/mL) quantification of mouse and human wild-type and pS129 α-synuclein protein. We then employed these assays, together with the BioLegend α-synuclein aggregate ELISA, to assess α-synuclein S129 phosphorylation and aggregation in different mouse brain tissue preparations. Overall, we highlight the compatibility of these new immunoassays with rodent models and demonstrate their potential to advance knowledge surrounding α-synuclein phosphorylation and aggregation in synucleinopathies.
© 2024. The Author(s).
Conflict of interest statement
N.D. is an Associate Editor of npj Parkinson’s Disease. N.D. was not involved in the journal’s review of, or decisions related to, this manuscript. The authors declare no other competing interests.
Figures



References
-
- Halliday, G. M., Holton, J. L., Revesz, T. & Dickson, D. W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol.122, 187–204 (2011). - PubMed
-
- Wakabayashi, K. et al. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol.47, 495–508 (2013). - PubMed
-
- Arima, K. et al. Immunoelectron-microscopic demonstration of NACP/alpha-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson’s disease and in dementia with Lewy bodies. Brain Res.808, 93–100 (1998). - PubMed
-
- Shahmoradian, S. H. et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci.22, 1099–1109 (2019). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

