Energy-transfer-enabled photocatalytic transformations of aryl thianthrenium salts
- PMID: 39516492
- PMCID: PMC11549398
- DOI: 10.1038/s41467-024-54079-3
Energy-transfer-enabled photocatalytic transformations of aryl thianthrenium salts
Abstract
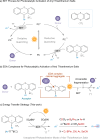
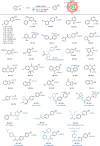
Aryl thianthrenium salts are valuable in photocatalysis but traditionally require external electron donors for activation. This study introduces an energy transfer (EnT) strategy for the activation of aryl thianthrenium salts using 2,3,4,5,6-penta(carbazol-9-yl)benzonitrile (5CzBN) as a metal-free photocatalyst, eliminating the need for external donors. Utilizing this EnT approach, we achieve C-H deuteration of arenes under visible light with CDCl3 as a deuterium source to synthesize various deuterated aromatic compounds, including important natural products and pharmaceuticals. Additionally, this strategy enables diverse functionalizations including borylation, arylation, cyanation, and selenylation, enhancing the applicability of aryl sulfonium salts in environmentally friendly photocatalysis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Visible-light-catalyzed C-H arylation of (hetero)arenes via arylselenonium salts.Org Biomol Chem. 2022 Jun 1;20(21):4427-4430. doi: 10.1039/d2ob00507g. Org Biomol Chem. 2022. PMID: 35587033
-
Visible-Light-Mediated Copper-Catalyzed S-Arylation of Sulfenamides with Aryl Thianthrenium Salts.Org Lett. 2025 May 16;27(19):4886-4892. doi: 10.1021/acs.orglett.5c01145. Epub 2025 May 2. Org Lett. 2025. PMID: 40314649
-
Visible-Light-Mediated Synthesis of Anomeric S-Aryl Glycosides via Electron Donor-Acceptor Complex Using Thianthrenium Salts.Molecules. 2025 Mar 14;30(6):1315. doi: 10.3390/molecules30061315. Molecules. 2025. PMID: 40142087 Free PMC article.
-
Photoactivation of Thianthrenium Salts: An Electron-Donor-Acceptor (EDA)-Complex Approach.J Org Chem. 2025 May 23;90(20):6617-6643. doi: 10.1021/acs.joc.5c00194. Epub 2025 May 14. J Org Chem. 2025. PMID: 40368878 Review.
-
Organothianthrenium salts: synthesis and utilization.Chem Sci. 2022 Oct 7;13(46):13690-13707. doi: 10.1039/d2sc04507a. eCollection 2022 Nov 30. Chem Sci. 2022. PMID: 36544727 Free PMC article. Review.
Cited by
-
Pd-catalyzed deuteration of aryl halides with deuterium oxide.Nat Commun. 2025 Mar 16;16(1):2584. doi: 10.1038/s41467-025-57855-x. Nat Commun. 2025. PMID: 40089487 Free PMC article.
-
Harvesting singlet and triplet excitation energies in covalent organic frameworks for highly efficient photocatalysis.Nat Mater. 2025 Aug;24(8):1245-1257. doi: 10.1038/s41563-025-02281-z. Epub 2025 Jul 16. Nat Mater. 2025. PMID: 40670709
-
Photocatalyst-free photochemical deuteration via H/D exchange with D2O.Nat Commun. 2025 Jul 22;16(1):6744. doi: 10.1038/s41467-025-61641-0. Nat Commun. 2025. PMID: 40695788 Free PMC article.
-
Thiol-free arene C-H thioesterification enabled by a photoactive electron donor-acceptor complex.Chem Sci. 2025 Aug 12. doi: 10.1039/d5sc05002b. Online ahead of print. Chem Sci. 2025. PMID: 40852456 Free PMC article.
-
Hydrogen Bond Promoted Carbonylative Lactonization of Alkenes.Chemistry. 2025 Apr 15;31(22):e202500487. doi: 10.1002/chem.202500487. Epub 2025 Mar 18. Chemistry. 2025. PMID: 40038050 Free PMC article.
References
-
- Meerwein, H., Büchner, E. & van Emster, K. Über die Einwirkung aromatischer Diazoverbindungen auf α,β-ungesättigte Carbonylverbindungen. J. Prakt. Chem.152, 237–266 (1939).
-
- Hari, D. P. & König, B. The photocatalyzed meerwein arylation: Classic reaction of aryl diazonium salts in a new light. Angew. Chem. Int. Ed.52, 4734–4743 (2013). - PubMed
-
- Ghosh, I., Marzo, L., Das, A., Shaikh, R. & König, B. Visible light mediated photoredox catalytic arylation reactions. Acc. Chem. Res.49, 1566–1577 (2016). - PubMed
-
- Galli, C. Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical. Chem. Rev.88, 765–792 (1988).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

