Randomized phase II trial of weekly ixabepilone ± biweekly bevacizumab for platinum-resistant or refractory ovarian/fallopian tube/primary peritoneal cancer (NCT03093155): Updated survival and subgroup analyses
- PMID: 39516558
- PMCID: PMC11523995
- DOI: 10.1038/s44276-024-00067-5
Randomized phase II trial of weekly ixabepilone ± biweekly bevacizumab for platinum-resistant or refractory ovarian/fallopian tube/primary peritoneal cancer (NCT03093155): Updated survival and subgroup analyses
Abstract
Background: Ixabepilone may retain activity in paclitaxel-resistant disease. We previously reported improved response rates (ORR), progression-free (PFS), and overall survival (OS) conferred by ixabepilone+bevacizumab (IXA + BEV) compared to monotherapy (IXA) in heavily pre-treated ovarian cancers. We now describe a mature data set. Subset analyses were performed in patients with different taxane sensitivities and dose modifications.
Methods: Patients previously treated with paclitaxel were stratified by prior BEV and randomized to receive IXA 20 mg/m2 days 1,8,15 ± BEV 10 mg/kg days 1,15 of a 28-day cycle in a multi-site prospective randomized phase 2 trial.
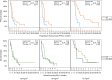
Results: Thirty-seven patients were randomized to IXA and 39 patients to IXA + BEV. At the final data cutoff (05/27/2023), ORR was higher in the IXA + BEV arm (38.4% vs. 8.1%, p = 0.003). Dose reductions were necessary in most participants but did not diminish PFS/OS benefits. Most patients were paclitaxel-refractory/-resistant (51%, n = 19/37;67%, n = 26/39); the remainder were taxane-sensitive. The addition of BEV to IXA conferred benefit in PFS (5.5 vs. 2.2 mo; HR 0.31, 90%CI 0.20-0.49, p < 0.001) and OS (10.3 vs. 6.0 mo; HR 0.56, 90%CI 0.38-0.84, p = 0.02) that persisted after adjusting for prior taxane response.
Conclusions: IXA + BEV has activity in heavily pre-treated ovarian cancers and offers significant improvement in ORR and PFS/OS compared to IXA, despite prior taxane response and dose reductions.
Clinical trial registration: NCT03093155.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003;21:3194–200. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Ovarian, Fallopian Tube, Primary Peritoneal Cancer v2.2023. 2023. Available from: https://www.nccn.org/
-
- Poveda AM, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, et al. Bevacizumab combined with weekly Paclitaxel, Pegylated Liposomal Doxorubicin, or Topotecan in Platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized Phase III AURELIA trial. J Clin Oncol 2015;33:3836–8,. - PubMed
-
- Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J, Clin Oncol 2014;32:1302–8. - PubMed
-
- Kessous R, Wissing MD, Laskov I, Abitbol J, Bitharas J, Agnihotram VR, et al. Multiple lines of chemotherapy for patients with high-grade ovarian cancer: Predictors for response and effect on survival. Int J Cancer. 2021;148:2304–12. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
