Temporal evolution and inter-patient heterogeneity in primary and recurrent head and neck squamous cell carcinoma
- PMID: 39516649
- PMCID: PMC11524138
- DOI: 10.1038/s44276-024-00091-5
Temporal evolution and inter-patient heterogeneity in primary and recurrent head and neck squamous cell carcinoma
Abstract
Background: Head and neck squamous cell carcinomas (HNSCCs) are heterogeneous in terms of origin and aetiology. In addition, there is uncertainty about the genetic evolution from initial diagnosis to recurrence after primary treatments and further disease progression following systemic treatment. Changes in the genetic profile have implications on the selection of appropriate treatments for patients, especially in the era of targeted therapies and immunotherapies.
Methods: We analysed a cohort of nine HNSCC patients with metachronous recurrence. All patients had paired primary and recurrent samples suitable for whole-exome sequencing, while transcriptomic data from seven patients could be analysed (multiple recurrent samples collected at different time points were available for three patients).
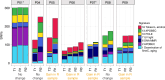
Results: At the genomic level, the recurrences shared a fraction of the somatic single nucleotide variants (SNVs) with the index primary tumours, but they also acquired many additional mutations, while losing only a few others. A similar behaviour was also observed when examining the changes of mutational signatures between primary and recurrent samples. Overall, recurrences appeared thus more genetically diverse than the respective primary tumours. The transcriptomic analysis showed that recurrent samples had lower immune cell presence, which was also confirmed by the multiplex immunofluorescence (IF) histology assays performed on the PhenoCycler platform. Several genes related to immune response were significantly downregulated compared to the primary samples.
Conclusions: Our results underline the importance of analysing multiple samples per patient to obtain a more complete picture of the patient's tumour and advocate a re-biopsy in the event of recurrence and treatment failure, in order to select the most appropriate therapeutic strategy.
© 2024. The Author(s).
Conflict of interest statement
US and VB are employees at BioNTech SE. US is co-founder and shareholder of TRON, co-founder and CEO of BioNTech SE. AK received fees for consulting, advisory, speaker’s roles and/or research funding from PUMA BioTechnology, AstraZeneca, Merck, MSD, Bristol-Myers Squibb, and Avvinity Therapeutics. HM has personal financial interests with AstraZeneca, MSD, GSK, Sanofi Pasteur, Merck, Warwickshire Head Neck Clinic Ltd; institutional financial interests with AstraZeneca, GSK PLC, Sanofi Pasteur, MSD, GSK Biologicals, Silence Therapeutics; leadership roles: Chief of Liteform Trial Steering Committee, Chair of NIMRAD Trial Steering Committee, President of the British Association of Head Neck Oncologists, Trial Steering Group member of the MRC CTU Cancer Trials Steering Committee, Council member of the International Association of Oral Oncology, Director of the Institute for Head Neck Studies and Education, Secretary of the Head Neck Cancer InterGroup. HM is a National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. All other authors have no conflict of interest.
Figures



References
-
- Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–54. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
