Validation of a guidelines-based digital tool to assess the need for germline cancer genetic testing
- PMID: 39516903
- PMCID: PMC11545665
- DOI: 10.1186/s13053-024-00298-0
Validation of a guidelines-based digital tool to assess the need for germline cancer genetic testing
Abstract
Background: Efficient and scalable solutions are needed to identify patients who qualify for germline cancer genetic testing. We evaluated the clinical validity of a brief, patient-administered hereditary cancer risk assessment digital tool programmed to assess if patients meet criteria for germline genetic testing, based on personal and family history, and in line with national guidelines.
Methods: We applied the tool to cases seen in a nationwide telehealth genetic counseling practice. Validity of the tool was evaluated by comparing the tool's assessment to that of the genetic counselor who saw the patient. Patients' histories were extracted from genetic counselor-collected pedigrees and input into the tool by the research team to model how a patient would complete the tool. We also validated the tool's assessment of which specific aspects of the personal and family history met criteria for genetic testing. Descriptive statistics were used.
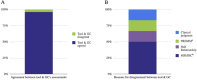
Results: Of the 152 cases (80% female, mean age 52.3), 56% had a personal history of cancer and 66% met genetic testing criteria. The tool and genetic counselor agreed in 96% of cases. Most disagreements (4/6; 67%) occurred because the genetic counselor's assessment relied on details the tool was not programmed to collect since patients typically don't have access to the relevant information (pathology details, risk models). We also found complete agreement between the tool and research team on which specific aspects of the patient's history met criteria for genetic testing.
Conclusion: We observed a high level of agreement with genetic counselor assessments, affirming the tool's clinical validity in identifying individuals for hereditary cancer predisposition testing and its potential for increasing access to hereditary cancer risk assessment.
Keywords: Cancer risk assessment; Genetic testing; Hereditary cancer.
© 2024. The Author(s).
Conflict of interest statement
CR, AD, DV, AH, KJ, JD, HC, CA, MS, CC declare employment at Genome Medical; AD, DV, AH, KJ, JD, HC, CA, MS, CC declare stock and/or stock options in Genome Medical; KJ, JD, MS declare leadership in Genome Medical; CR, AD, DV, AH, KJ, JD, KR, CA, MS, CC declare a patent pending on technology underpinning the digital tool that is the focus of this article; KR declares stock options in Stata Oncology; DV declares employment, ownership interest, and consulting with Call Light Health.
Figures
References
-
- US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Risk Assessment, genetic counseling, and genetic testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652–65. - PubMed
-
- Parente DJ. BRCA-Related Cancer Genetic Counseling is indicated in many women seeking primary care. J Am Board Fam Med. 2020;33:885–93. - PubMed
-
- Linfield DT, Rothberg MB, Pfoh ER, Noss R, Cassard L, Powers JC, et al. Primary care physician referral practices regarding BRCA1/2 genetic counseling in a major health system. Breast Cancer Res Treat. 2022;195:153–60. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

