Melatonin supplementation attenuates cuproptosis and ferroptosis in aging cumulus and granulosa cells: potential for improving IVF outcomes in advanced maternal age
- PMID: 39516964
- PMCID: PMC11545199
- DOI: 10.1186/s12958-024-01311-w
Melatonin supplementation attenuates cuproptosis and ferroptosis in aging cumulus and granulosa cells: potential for improving IVF outcomes in advanced maternal age
Abstract
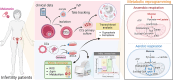
Background: Advanced maternal age is associated with decreased oocyte quantity and quality and in vitro fertilization (IVF) success rates. This study aimed to investigate whether melatonin supplementation can improve IVF outcomes in women of advanced maternal age by modulating cuproptosis and ferroptosis.
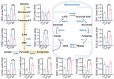
Methods: This prospective cohort study included 161 women aged 35-45 years undergoing IVF-frozen embryo transfer cycles. Participants were assigned to either melatonin (n = 86, 2 mg daily for ≥ 8 weeks) or control (n = 75) groups. Cumulus cells were analyzed for cuproptosis and ferroptosis-related gene expression. Additional experiments were conducted on the HGL5 human granulosa cell line to assess mitochondrial function and metabolic reprogramming.
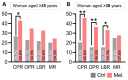
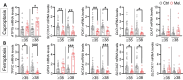
Results: Melatonin supplementation significantly improved IVF outcomes in women aged ≥ 38 years, increasing clinical pregnancy rates (46.0% vs. 20.3%, P < 0.01), ongoing pregnancy rates (36.5% vs. 15.3%, P < 0.01), and live birth rates (33.3% vs. 15.3%, P < 0.05). In cumulus cells from patients, gene expression analysis revealed that melatonin modulated cuproptosis and ferroptosis-related genes, including ATP7B and GPX4, with more pronounced effects in the ≥ 38 years group. This suggests melatonin enhances cellular resilience against oxidative stress and metal-induced toxicity in the ovarian microenvironment. In vitro studies using HGL5 cells showed melatonin reduced oxidative stress markers, improved mitochondrial function, restored expression of glycolysis and TCA cycle-related genes and modulated cuproptosis and ferroptosis-related gene expression. These findings provide mechanistic insight into melatonin's protective effects against regulated cell death in ovarian cells, potentially explaining the improved IVF outcomes observed.
Conclusions: Melatonin supplementation significantly improved IVF outcomes in women of advanced maternal age, particularly those ≥ 38 years old, likely by modulating cuproptosis and ferroptosis and enhancing mitochondrial function in cumulus and granulosa cells. These results suggest that melatonin could be a promising adjuvant therapy for improving IVF success rates in older women.
Keywords: Antioxidant therapy; Assisted reproduction; Mitochondrial function; Oocyte quality; Oxidative stress; Reproductive aging.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Mitochondrial SIRT3 and its target glutamate dehydrogenase are altered in follicular cells of women with reduced ovarian reserve or advanced maternal age.Hum Reprod. 2014 Jul;29(7):1490-9. doi: 10.1093/humrep/deu071. Epub 2014 Apr 25. Hum Reprod. 2014. PMID: 24771001
-
The effect of oral melatonin supplementation on MT-ATP6 gene expression and IVF outcomes in Iranian infertile couples: a nonrandomized controlled trial.Naunyn Schmiedebergs Arch Pharmacol. 2021 Jul;394(7):1487-1495. doi: 10.1007/s00210-021-02071-9. Epub 2021 Mar 8. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33683419 Clinical Trial.
-
Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS?Reprod Biomed Online. 2013 Jan;26(1):22-9. doi: 10.1016/j.rbmo.2012.10.007. Epub 2012 Oct 12. Reprod Biomed Online. 2013. PMID: 23177415 Clinical Trial.
-
Guidelines for the number of embryos to transfer following in vitro fertilization No. 182, September 2006.Int J Gynaecol Obstet. 2008 Aug;102(2):203-16. doi: 10.1016/j.ijgo.2008.01.007. Int J Gynaecol Obstet. 2008. PMID: 18773532 Review.
-
Inositol for subfertile women with polycystic ovary syndrome.Cochrane Database Syst Rev. 2018 Dec 20;12(12):CD012378. doi: 10.1002/14651858.CD012378.pub2. Cochrane Database Syst Rev. 2018. PMID: 30570133 Free PMC article.
Cited by
-
Melatonin Interplay in Physiology and Disease-The Fountain of Eternal Youth Revisited.Biomolecules. 2025 May 8;15(5):682. doi: 10.3390/biom15050682. Biomolecules. 2025. PMID: 40427575 Free PMC article. Review.
-
Melatonin biosynthesis and regulation in reproduction.Front Endocrinol (Lausanne). 2025 Jul 28;16:1630164. doi: 10.3389/fendo.2025.1630164. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40791993 Free PMC article. Review.
-
Mitochondrial dysfunction in oocytes: implications for fertility and ageing.J Ovarian Res. 2025 Aug 14;18(1):186. doi: 10.1186/s13048-025-01764-6. J Ovarian Res. 2025. PMID: 40814060 Free PMC article. Review.
-
Ovarian Endometrioma Disrupts Oocyte-Cumulus Communication and Mitochondrial Function, With Melatonin Mitigating the Effects.Cell Prolif. 2025 Apr;58(4):e13800. doi: 10.1111/cpr.13800. Epub 2025 Jan 21. Cell Prolif. 2025. PMID: 39837534 Free PMC article.
-
Oocyte and dietary supplements: a mini review.Front Cell Dev Biol. 2025 Jun 26;13:1619758. doi: 10.3389/fcell.2025.1619758. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40641606 Free PMC article. Review.
References
-
- Charalambous C, Webster A, Schuh M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat Rev Mol Cell Biol. 2023;24(1):27–44. - PubMed
-
- Owen A, Carlson K, Sparzak PB. Age-related fertility decline. StatPearls. Treasure Island (FL) companies. Disclosure: Karen Carlson declares no relevant financial relationships with ineligible companies. Disclosure: Paul Sparzak declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024. StatPearls Publishing LLC.; 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

