COVID-19 and severe cutaneous allergic reactions to sulfonamides
- PMID: 39517080
- PMCID: PMC11572944
- DOI: 10.2500/aap.2024.45.240086
COVID-19 and severe cutaneous allergic reactions to sulfonamides
Abstract
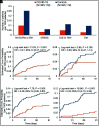
Background: Sulfonamides are associated with severe cutaneous adverse reactions (SCARs). Coronavirus disease 2019 (COVID-19) triggers an immune response, which may increase the likelihood of developing a hypersensitivity reaction. Objectives: We sought to explore the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on the probability of developing SCARs and/or erythema multiforme (EM) reactions to sulfonamides. Methods: In the propensity score-matched cohort study by using the de-identified TriNetX Research data base, patients who had an exposure to antibiotic or non-antibiotic sulfonamides between March 1, 2020, and January 1, 2023, were divided into two groups based on the presence or absence of a previous COVID-19 infection within 6 months of starting the sulfonamide agent. The outcomes studied were the 30-day risk of developing SCARs or EM (Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, or EM) within 3 months of sulfonamide exposure. Cohorts were matched based on baseline demographics; malignant lymphoid neoplasms; human immunodeficiency virus; systemic lupus erythematosus; bone marrow transplantation; diabetes; psoriasis; seizures; gout; solid organ or stem cell transplantation; COVID-19 vaccination; and exposure to risk medications, including allopurinol, levetiracetam, carbamazepine, lamotrigine, oxcarbazepine, phenytoin, phenobarbital, abacavir, nevirapine, piroxicam, tenoxicam, or mexiletine. Results: When comparing 345,119 patients on sulfonamides and with previous COVID-19 to an equal number of sulfonamides users without a previous COVID-19, patients with COVID-19 had a lower risk of developing any form of SCARs (relative risk 0.39 [95% confidence interval, 0.26, 0.58]; p < 0.001). Conclusion: Previous SARS-CoV-2 infection seems to be associated with a lower probability of developing SCARs or EM among patients using sulfonamides.
Conflict of interest statement
T.J. Craig served as a speaker and researcher for Biomarin, CSL-Behring, Gfrifols, Kalvista, and Takeda; researcher for Astria, Biomarin, Intellia, Ionis, KalVista, GSK, and Pharvaris; consultant for Astria, Biocryst, Biomarin, CSL Behring, Intellia, and Kalvista; director for ACARE International Hereditary Angioedema Center and Alpha-1 Resource Center; and member of the Medical Advisory Board for the Hereditary Angioedema Association. The other authors have no conflicts of interest to declare pertaining to this article
Figures
Similar articles
-
The association between carbamazepine and valproate and adverse cutaneous drug reactions in patients with bipolar disorder: a nested matched case-control study.J Clin Psychopharmacol. 2008 Oct;28(5):509-17. doi: 10.1097/JCP.0b013e3181845610. J Clin Psychopharmacol. 2008. PMID: 18794645
-
Severe cutaneous adverse reactions: A 5-year retrospective study at Hospital Melaka, Malaysia, from December 2014 to February 2020.Med J Malaysia. 2022 Jul;77(4):409-414. Med J Malaysia. 2022. PMID: 35902928
-
Sulfonamide Hypersensitivity.Clin Rev Allergy Immunol. 2022 Jun;62(3):400-412. doi: 10.1007/s12016-021-08872-3. Epub 2021 Jul 1. Clin Rev Allergy Immunol. 2022. PMID: 34212341 Review.
-
[Stevens-Johnson syndrome and toxic epidermal necrolysis following intake of sulfonamides].Hautarzt. 1982 Sep;33(9):484-90. Hautarzt. 1982. PMID: 7174320 German.
-
Erythema Multiforme and COVID-19: What Do We Know?Medicina (Kaunas). 2021 Aug 16;57(8):828. doi: 10.3390/medicina57080828. Medicina (Kaunas). 2021. PMID: 34441034 Free PMC article. Review.
Cited by
-
Toxic epidermal necrolysis following lamotrigine replacement therapy in a woman planning pregnancy: a case report and literature review.BMC Womens Health. 2025 Jul 28;25(1):371. doi: 10.1186/s12905-025-03796-y. BMC Womens Health. 2025. PMID: 40717110 Free PMC article. Review.
-
Integrating innovation and shared decision-making in allergy and immunology practice.Allergy Asthma Proc. 2024 Nov 1;45(6):395-397. doi: 10.2500/aap.2024.45.240089. Allergy Asthma Proc. 2024. PMID: 39517073 Free PMC article. No abstract available.
References
-
- Hafsi W, Badri T. Erythema Multiforme. In StatPearls. [internet]. StatPearls Publishing. 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

