PTP1B Modulates Carotid Plaque Vulnerability in Atherosclerosis Through Rab5-PDGFRβ-Mediated Endocytosis Disruption and Apoptosis
- PMID: 39517122
- PMCID: PMC11549062
- DOI: 10.1111/cns.70071
PTP1B Modulates Carotid Plaque Vulnerability in Atherosclerosis Through Rab5-PDGFRβ-Mediated Endocytosis Disruption and Apoptosis
Abstract
Background: Protein tyrosine phosphatase 1B (PTP1B) is a protein tyrosine phosphatase and modulates platelet-derived growth factor (PDGF)/platelet-derived growth factor receptor (PDGFR) signaling in vascular smooth muscle cells (VSMCs) via endocytosis. However, the related molecular pathways that participated in the interaction of endo-lysosome and the trafficking of PDGFR are largely unknown. This study aims to determine the subcellular regulating mechanism of PTP1B to the endo-lysosome degradation of PDGFR in atherosclerotic carotid plaques, thereby offering a potential therapeutic target for the stabilization of carotid plaques.
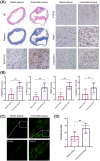
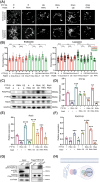
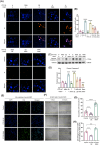
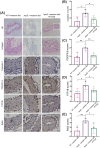
Methods: The immunohistochemical staining technique was employed to assess the expression levels of both PDGFR-β and Caspase 3 in stable and vulnerable carotid plaques. Tunnel staining was utilized to quantify the apoptosis of carotid plaques. Live-cell imaging was employed to observe endocytic motility, while cell apoptosis was evaluated through Propidium Iodide staining. In an in vivo experiment, ApoE-/- mice were administered a PTP1B inhibitor to investigate the impact of PTP1B on atherosclerosis.
Results: The heightened expression of PDGFR-β correlates with apoptosis in patients with vulnerable carotid plaques. At the subcellular level of VSMCs, PDGFR-β plays a pivotal role in sustaining a balanced endocytosis system motility, regulated by the expression of Rab5, a key regulator of endocytic motility. And PTP1B modulates PDGFR-β signaling via Rab5-mediated endocytosis. Additionally, disrupted endocytic motility influences the interplay between endosomes and lysosomes, which is crucial for controlling PDGFR-β trafficking. Elevated PTP1B expression induces cellular apoptosis and impedes migration and proliferation of carotid VSMCs. Ultimately, mice with PTP1B deficiency exhibit a reduction in atherosclerosis.
Conclusion: Our results illustrate that PTP1B induces disruption in endocytosis and apoptosis of VSMCs through the Rab5-PDGFRβ pathway, suggesting a potential association with the heightened vulnerability of carotid plaques.
Keywords: PTP1B; apoptosis; atherosclerosis; carotid plaque; endocytosis disruption.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Gao P., Wang T., Wang D., et al., “Effect of Stenting Plus Medical Therapy vs Medical Therapy Alone on Risk of Stroke and Death in Patients With Symptomatic Intracranial Stenosis: The CASSISS Randomized Clinical Trial,” Journal of the American Medical Association 328, no. 6 (2022): 534–542. - PMC - PubMed
-
- Brinjikji W., J. Huston, 3rd , Rabinstein A. A., Kim G. M., Lerman A., and Lanzino G., “Contemporary Carotid Imaging: From Degree of Stenosis to Plaque Vulnerability,” Journal of Neurosurgery 124, no. 1 (2016): 27–42. - PubMed
-
- Bentzon J. F., Otsuka F., Virmani R., and Falk E., “Mechanisms of Plaque Formation and Rupture,” Circulation Research 114, no. 12 (2014): 1852–1866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

