Enhanced Experimental Setup and Methodology for the Investigation of Corrosion Fatigue in Metallic Biodegradable Implant Materials
- PMID: 39517421
- PMCID: PMC11547594
- DOI: 10.3390/ma17215146
Enhanced Experimental Setup and Methodology for the Investigation of Corrosion Fatigue in Metallic Biodegradable Implant Materials
Abstract
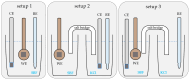
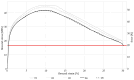
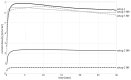
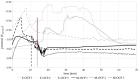
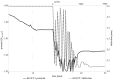
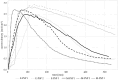
Biodegradable implants as bone fixations may present a safe alternative to traditional permanent implants, reducing the risk of infections, promoting bone healing, and eliminating the need for removal surgeries. Structural integrity is an important consideration when choosing an implant material. As a biodegradable implant is being resorbed, until the natural bone has regrown, the implant material needs to provide mechanical stability. However, the corrosive environment of the human body may affect the fatigue life of the material. Conversely, mechanical stress can have an effect on electrochemical corrosion processes. This is known as corrosion fatigue. In the presented work, an experimental setup and methodology was established to analyze the corrosion fatigue of experimental bioresorbable materials while simultaneously monitoring the electrochemical processes. A double-walled measurement cell was constructed for a three-point bending test in Dulbecco's Phosphate-Buffered Saline (DPBS- -), which was used as simulated body fluid (SBF), at 37 ± 1 °C. The setup was combined with a three-electrode setup for corrosion measurements. Rod-shaped zinc samples were used to validate the setup's functionality. Preliminary static and dynamic bending tests were carried out as per the outlined methodology to determine the test parameters. Open-circuit as well as potentiostatic polarization measurements were performed with and without mechanical loading. For the control, fatigue tests were performed in an air environment. The tested zinc samples were inspected via scanning electron microscopy (SEM). Based on the measured mechanical and electrochemical values as well as the SEM images, the effects of the different environments were investigated, and the setup's functionality was verified. An analysis of the data showed that a comprehensive investigation of corrosion fatigue characteristics is feasible with the outlined approach. Therefore, this novel methodology shows great potential for furthering our understanding of the effects of corrosion on the fatigue of biodegradable implant materials.
Keywords: biodegradable implants; corrosion fatigue; experimental setup; open-circuit potential; potentiostatic polarization; three-point bending test; zinc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. - DOI - PMC - PubMed
-
- Jahr H., Li Y., Zhou J., Zadpoor A.A., Schröder K.-U. Additively Manufactured Absorbable Porous Metal Implants—Processing, Alloying and Corrosion Behavior. Front. Mater. 2021;8:628633. doi: 10.3389/fmats.2021.628633. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

