Implementing the Design of Experiments (DoE) Concept into the Development of Mucoadhesive Tablets Containing Orange Peel Extract as a Potential Concept for the Treatment of Oral Infections
- PMID: 39517510
- PMCID: PMC11547214
- DOI: 10.3390/ma17215234
Implementing the Design of Experiments (DoE) Concept into the Development of Mucoadhesive Tablets Containing Orange Peel Extract as a Potential Concept for the Treatment of Oral Infections
Abstract
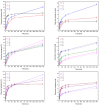
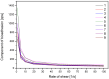
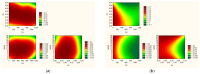
This study explores for the first time the impact of chitosan (CS) with varying molecular weights (MW), orange peel extract concentration, and hydroxypropyl methylcellulose (HPMC) content on the formulation of buccal tablets for treating oral infections. Utilizing a statistical design of experiments (DoE), nine different formulations were evaluated for mechanical properties, dissolution behavior, mucoadhesion, and biological activity. A formulation with high CS MW, 60% orange peel extract, and 8% HPMC, emerged as the optimal formulation, demonstrating superior tabletability, compressibility, and compactibility. Dissolution studies indicated that hesperidin release followed the Higuchi model, with higher extract content enhancing this phenomenon. Mucoadhesion improved with increased HPMC and CS concentrations, although higher extract content reduced bioadhesion. Biological assays showed that higher extract levels boosted antioxidant activity, while CS primarily contributed to anti-inflammatory effects. The optimized formulation exhibited broad antimicrobial activity against key oral pathogens, surpassing the effectiveness of the individual components. Principal component analysis (PCA) further confirmed the significant influence of extract content on tablet properties. These findings suggest that the optimized tablet formulation holds promise for effective buccal delivery in the treatment of oral infections, warranting further investigation in clinical settings.
Keywords: buccal tablets; chitosan; dissolution; hydroxypropyl methylcellulose; mucoadhesion; oral cavity infections; orange peel extract; statistical design of experiments.
Conflict of interest statement
Author Michał Walendowski was employed by the company Science-Bridge Sp. z o.o. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Optimization of piribedil mucoadhesive tablets for efficient therapy of Parkinson's disease: physical characterization and ex vivo drug permeation through buccal mucosa.Drug Dev Ind Pharm. 2017 Nov;43(11):1836-1845. doi: 10.1080/03639045.2017.1349785. Epub 2017 Jul 12. Drug Dev Ind Pharm. 2017. PMID: 28665152
-
Mucoadhesive buccal tablet of leuprolide and its fatty acid conjugate: Design, in vitro evaluation and formulation strategies.Int J Pharm. 2023 May 25;639:122963. doi: 10.1016/j.ijpharm.2023.122963. Epub 2023 Apr 15. Int J Pharm. 2023. PMID: 37068715
-
Mucoadhesive bilayered buccal platform for antifungal drug delivery into the oral cavity.Drug Deliv Transl Res. 2021 Feb;11(1):318-327. doi: 10.1007/s13346-020-00798-1. Drug Deliv Transl Res. 2021. PMID: 32578045
-
It is Possible to Achieve Tablets With Good Tabletability From Solid Dispersions - The Case of the High Dose Drug Gemfibrozil.Curr Drug Deliv. 2021;18(4):460-470. doi: 10.2174/1567201817666201023121948. Curr Drug Deliv. 2021. PMID: 33100203
-
Influence of hydroxypropyl methylcellulose mixture, apparent viscosity, and tablet hardness on drug release using a 2(3) full factorial design.Drug Dev Ind Pharm. 2002 May;28(5):601-8. doi: 10.1081/ddc-120003456. Drug Dev Ind Pharm. 2002. PMID: 12098849
References
-
- Oral Health. [(accessed on 19 April 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health.
-
- Paczkowska-Walendowska M., Dvořák J., Rosiak N., Tykarska E., Szymańska E., Winnicka K., Ruchała M.A., Cielecka-Piontek J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics. 2021;13:417. doi: 10.3390/pharmaceutics13030417. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

