Synthesis and Thermal Properties of Bio-Based Janus Ring Siloxanes Incorporating Terpenes and Terpenoids
- PMID: 39517618
- PMCID: PMC11547749
- DOI: 10.3390/ma17215348
Synthesis and Thermal Properties of Bio-Based Janus Ring Siloxanes Incorporating Terpenes and Terpenoids
Abstract
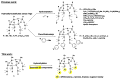
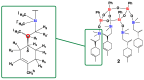
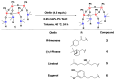
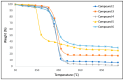
A mild and highly selective hydrosilylation method was employed to synthesize five novel well-defined Janus ring siloxanes bearing terpenes and terpenoids, which are the main bioactive components of essential oils. The characterization of these new bio-sourced molecular materials, derived from hydrosilyl-substituted all-cis-cyclotetrasiloxane, was conducted through comprehensive analyses using multinuclear NMR, infrared spectroscopy, elemental analysis, and mass spectroscopy. The thermal stability of the newly synthesized Janus rings was investigated, and the siloxane skeleton was shown to confer an enhanced thermal stability compared with free terpenes and terpenoids.
Keywords: Janus ring siloxane; all-cis-cyclotetrasiloxane; hydrosilylation; silsesquioxane; terpenes; terpenoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
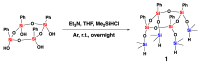

References
-
- Baney R.H., Itoh M., Sakakibara A., Suzuki T. Silsesquioxanes. Chem. Rev. 1995;95:1409–1430. doi: 10.1021/cr00037a012. - DOI
-
- Cordes D.B., Lickiss P.D., Rataboul F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxane. Chem. Rev. 2010;110:2081–2173. - PubMed
-
- Cordes D.B., Lickiss P.D. Preparation and Characterization of Polyhedral Oligosilsesquioxanes. In: Matisons J., Hartmann-Thompson C., editors. Applications of Polyhedral Oligomeric Silsesquioxanes. Volume 3. Springer; Dordrecht, The Netherlands: 2011. pp. 47–133. (Advances in Silicon Science Series).
-
- Laine R.M., Roll M.F. Polyhedral phenylsilsesquioxanes. Macromolecules. 2011;44:1073–1109. doi: 10.1021/ma102360t. - DOI
-
- Zhou H., Ye Q., Xu J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017;1:212–230. doi: 10.1039/C6QM00062B. - DOI
LinkOut - more resources
Full Text Sources

