Cannabidiol (CBD) Protects Lung Endothelial Cells from Irradiation-Induced Oxidative Stress and Inflammation In Vitro and In Vivo
- PMID: 39518030
- PMCID: PMC11544820
- DOI: 10.3390/cancers16213589
Cannabidiol (CBD) Protects Lung Endothelial Cells from Irradiation-Induced Oxidative Stress and Inflammation In Vitro and In Vivo
Abstract
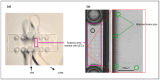
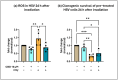
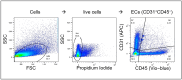
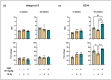
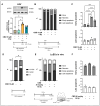
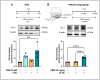
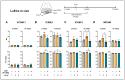
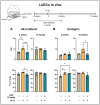
Objective: Radiotherapy, which is commonly used for the local control of thoracic cancers, also induces chronic inflammatory responses in the microvasculature of surrounding normal tissues such as the lung and heart that contribute to fatal radiation-induced lung diseases (RILDs) such as pneumonitis and fibrosis. In this study, we investigated the potential of cannabidiol (CBD) to attenuate the irradiation damage to the vasculature. Methods: We investigated the ability of CBD to protect a murine endothelial cell (EC) line (H5V) and primary lung ECs isolated from C57BL/6 mice from irradiation-induced damage in vitro and lung ECs (luECs) in vivo, by measuring the induction of oxidative stress, DNA damage, apoptosis (in vitro), and induction of inflammatory and pro-angiogenic markers (in vivo). Results: We demonstrated that a non-lethal dose of CBD reduces the irradiation-induced oxidative stress and early apoptosis of lung ECs by upregulating the expression of the cytoprotective mediator heme-oxygenase-1 (HO-1). The radiation-induced increased expression of inflammatory (ICAM-2, MCAM) and pro-angiogenic (VE-cadherin, Endoglin) markers was significantly reduced by a continuous daily treatment of C57BL/6 mice with CBD (i.p. 20 mg/kg body weight), 2 weeks before and 2 weeks after a partial irradiation of the lung (less than 20% of the lung volume) with 16 Gy. Conclusions: CBD has the potential to improve the clinical outcome of radiotherapy by reducing toxic side effects on the microvasculature of the lung.
Keywords: cannabidiol (CBD); inflammation; oxidative stress; radiation-induced lung disease; radiotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

