Rac1 GTPase Regulates the βTrCP-Mediated Proteolysis of YAP Independently of the LATS1/2 Kinases
- PMID: 39518045
- PMCID: PMC11545309
- DOI: 10.3390/cancers16213605
Rac1 GTPase Regulates the βTrCP-Mediated Proteolysis of YAP Independently of the LATS1/2 Kinases
Abstract
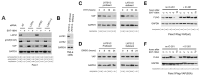
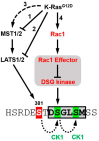
Background: Oncogenic mutations in the KRAS gene are detected in >90% of pancreatic cancers (PC). In genetically engineered mouse models of PC, oncogenic KRAS drives the formation of precursor lesions and their progression to invasive PC. The Yes-associated Protein (YAP) is a transcriptional coactivator required for transformation by the RAS oncogenes and the development of PC. In Ras-driven tumors, YAP can also substitute for oncogenic KRAS to drive tumor survival after the repression of the oncogene. Ras oncoproteins exert their transforming properties through their downstream effectors, including the PI3K kinase, Rac1 GTPase, and MAPK pathways. Methods: To identify Ras effectors that regulate YAP, YAP levels were measured in PC cells exposed to inhibitors of oncogenic K-Ras and its effectors. Results: In PC cells, the inhibition of Rac1 leads to a time-dependent decline in YAP protein, which could be blocked by proteosome inhibitor MG132. This YAP degradation after Rac1 inhibition was observed in a range of cell lines using different Rac1 inhibitors, Rac1 siRNA, or expression of dominant negative Rac1T17N mutant. Several E3 ubiquitin ligases, including SCFβTrCP, regulate YAP protein stability. To be recognized by this ligase, the βTrCP degron of YAP (amino acid 383-388) requires its phosphorylation by casein kinase 1 at Ser384 and Ser387, but these events must first be primed by the phosphorylation of Ser381 by LATS1/2. Using Flag-tagged mutants of YAP, we show that YAP degradation after Rac1 inhibition requires the integrity of this degron and is blocked by the silencing of βTrCP1/2 and by the inhibition of casein kinase 1. Unexpectedly, YAP degradation after Rac1 inhibition was still observed after the silencing of LATS1/2 or in cells carrying a LATS1/2 double knockout. Conclusions: These results reveal Rac1 as an oncogenic KRAS effector that contributes to YAP stabilization in PC cells. They also show that this regulation of YAP by Rac1 requires the SCFβTrCP ligase but occurs independently of the LATS1/2 kinases.
Keywords: GTPase; Hippo; Rac1; Ras; YAP; pancreatic cancer; ubiquitin ligases; βTrCP.
Conflict of interest statement
S.K.B. is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. Other authors declare that they have no conflicts of interest.
Figures



References
-
- Pellegata N.S., Sessa F., Renault B., Bonato M., Leone B.E., Solcia E., Ranzani G.N., Peracaula R., Cleary K.R., Lorenzo J., et al. K-ras and p53 gene mutations in pancreatic cancer. Cancer Res. 1994;54:1556–1560. - PubMed
-
- Hong S.M., Vincent A., Kanda M., Leclerc J., Omura N., Borges M., Klein A.P., Canto M.I., Hruban R.H., Goggins M. Genome-wide somatic copy number alterations in low-grade PanINs and IPMNs from individuals with a family history of pancreatic cancer. Clin. Cancer Res. 2012;18:4303–4312. doi: 10.1158/1078-0432.CCR-12-1075. - DOI - PMC - PubMed
-
- Kanda M., Matthaei H., Wu J., Hong S.M., Yu J., Borges M., Hruban R.H., Maitra A., Kinzler K., Vogelstein B., et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733.e739. doi: 10.1053/j.gastro.2011.12.042. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

