Sacituzumab Govitecan in Triple Negative Breast Cancer: A Systematic Review of Clinical Trials
- PMID: 39518062
- PMCID: PMC11545346
- DOI: 10.3390/cancers16213622
Sacituzumab Govitecan in Triple Negative Breast Cancer: A Systematic Review of Clinical Trials
Abstract
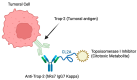
Background/objectives: Triple-negative breast cancer is difficult to treat due to the absence of hormone receptors and Her2neu. Sacituzumab govitecan is a new therapeutic approach that uses an antibody directed against the Trop-2 antigen present in solid epithelial tumors, linked to the active metabolite SN-38, similar to irinotecan, to specifically target cancer cells while minimizing damage to healthy cells. The objective of the present review was to evaluate the efficacy and safety of sacituzumab govitecan as a single treatment in patients with triple-negative breast cancer and to compare its results with the standard conventional chemotherapy regimen currently used in this disease.
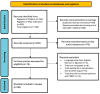
Methods: A systematic review of randomized clinical trials of sacituzumab govitecan was performed. The search was performed in Medline (PubMed), Web of Science, and Cochrane from September 2022 to January 2024.
Results: Thirty-eight articles are included and evaluated according to inclusion and exclusion criteria corresponding to the two most relevant clinical trials, including specific analyses of cohorts and subgroup study arms within these trials. Data from more recent clinical trials are also reviewed.
Conclusions: The efficacy results showed a significantly greater clinical benefit with sacituzumab govitecan compared to standard chemotherapy in patients with triple-negative breast cancer. This drug will become a treatment of substantial impact in future treatment guidelines for this type of cancer.
Keywords: Trop-2 antigen; UGT1A1; antibody–drug conjugate; bystander effect; chemotherapy; sacituzumab govitecan; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Arenas M., Fargas-Saladié M., Moreno-Solé M., Moyano-Femenia L., Jiménez-Franco A., Canela-Capdevila M., Castañé H., Martínez-Navidad C., Camps J., Joven J. Metabolomics and triple-negative breast cancer: A systematic review. Heliyon. 2023;10:e23628. doi: 10.1016/j.heliyon.2023.e23628. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous