Combined Radiotherapy and Hyperthermia: A Systematic Review of Immunological Synergies for Amplifying Radiation-Induced Abscopal Effects
- PMID: 39518094
- PMCID: PMC11545184
- DOI: 10.3390/cancers16213656
Combined Radiotherapy and Hyperthermia: A Systematic Review of Immunological Synergies for Amplifying Radiation-Induced Abscopal Effects
Abstract
Introduction: The abscopal effect is a systemic immune response characterized by metastases regression at sites distant from the irradiated lesion. This systematic review aims to explore the immunological mechanisms of action underlying the abscopal effect and to investigate how hyperthermia (HT) can increase the chances of radiotherapy (RT) triggering systemic anti-tumor immune responses.
Methods: This review is created in accordance with the PRISMA guidelines.
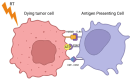
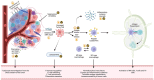
Results and conclusion: HT and RT have both complementary and synergistic immunological effects. Both methods trigger danger signal release, promoting cytokine and chemokine secretion, which increases T-cell infiltration and facilitates cell death. Both treatments upregulate extracellular tumor HSP70, which could amplify DAMP recognition by macrophages and DCs, leading to stronger tumor antigen presentation and CTL-mediated immune responses. Additionally, the combined increase in cell adhesion molecules (VCAM-1, ICAM-1, E-selectin, L-selectin) could enhance leukocyte adhesion to tumors, improving lymphocyte trafficking and boosting systemic anti-tumor effects. Lastly, HT causes vasodilation and improves blood flow, which might exacerbate those distant effects. We suggest the combination of local radiotherapy with fever-range whole-body hyperthermia to optimally enhance the chances of triggering the abscopal effect mediated by the immune system.
Keywords: cancer; hyperthermia; immune system; metastasis; radiotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

