Cutaneous Toxicities of Advanced Treatment for Cutaneous Melanoma: A Prospective Study from a Single-Center Institution
- PMID: 39518117
- PMCID: PMC11545238
- DOI: 10.3390/cancers16213679
Cutaneous Toxicities of Advanced Treatment for Cutaneous Melanoma: A Prospective Study from a Single-Center Institution
Abstract
Background/objectives: The landscape of advanced melanoma treatments has shifted dramatically in recent years. Target therapy and immunotherapy have changed the management of patients with both metastatic (stage IV according to AJCC 8th ed.) and nodal (stage IIB/C and III) disease. As the use of novel agents has increased, so have the cutaneous toxicities associated with these medications. While most skin reactions are low-grade and can be managed conservatively with topical therapies, high-grade or life-threatening drug reactions can arise during therapy, requiring prompt dermatologic recognition and treatment. Given the survival benefit attributed to these new agents, treating skin toxicity and maintaining a patient's quality of life is of paramount importance.
Methods: We undertook a prospective, monocentric, and descriptive study in Bologna, Italy, including patients referred to the Oncologic Dermatology Unit of IRCCS AOU of Bologna who developed biopsy-proven cutaneous adverse events (AE) under treatment with immunotherapy for cutaneous melanoma with nodal (stage IIB/C, III) and metastatic (stage IV) disease from January 2016 to April 2024.
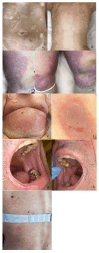
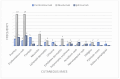
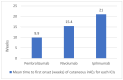
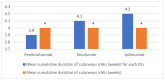
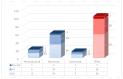
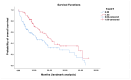
Results: In 202 identified patients, 75 (37.5%) developed skin AEs. Ipilimumab was causal for 48.1% of skin AEs, followed by nivolumab (37%) and pembrolizumab (31.4%). Recorded types of skin AEs included erythematous rash, vitiligo, alopecia, lichenoid, maculopapular, acneiform, urticarial, psoriasiform, granulomatous, eczematous, and severe cutaneous AEs, such as Erythema multiforme/Stevens-Johnson syndrome and bullous autoimmune dermatoses. Most AEs were low-grade [CTCAE 1-2] (97%) and typically occurred after 10 weeks of treatment.
Conclusions: This study comprehensively describes skin AEs occurring during systemic treatment with ICIs for cutaneous melanoma at a single center.
Keywords: cutaneous toxicity; immunotherapy; ipilimumab; melanoma; nivolumab; pembrolizumab; toxicity.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Matthews N.H., Li W.-Q., Qureshi A.A., Weinstock M.A., Cho E. Epidemiology of Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; Brisbane, Australia: 2017. - PubMed
-
- De Giorgi V., Magnaterra E., Zuccaro B., Magi S., Magliulo M., Medri M., Mazzoni L., Venturi F., Silvestri F., Tomassini G.M., et al. Is Pediatric Melanoma Really That Different from Adult Melanoma? A Multicenter Epidemiological, Clinical and Dermoscopic Study. Cancers. 2023;15:1835. doi: 10.3390/cancers15061835. - DOI - PMC - PubMed
-
- Dika E., Chessa M.A., Veronesi G., Ravaioli G.M., Fanti P.A., Ribero S., Tripepi G., Gurioli C., Traniello Gradassi A., Lambertini M., et al. A Single Institute’s Experience on Melanoma Prognosis: A Long-Term Follow-Up. G. Ital. Dermatol. Venereol. 2018;153:326–332. doi: 10.23736/S0392-0488.17.05420-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

