Enhancing Anti-PD-1 Immunotherapy by Targeting MDSCs via Hepatic Arterial Infusion in Breast Cancer Liver Metastases
- PMID: 39518148
- PMCID: PMC11545300
- DOI: 10.3390/cancers16213711
Enhancing Anti-PD-1 Immunotherapy by Targeting MDSCs via Hepatic Arterial Infusion in Breast Cancer Liver Metastases
Abstract
Background: Surgery, chemotherapy, and radiation often have limited utility for advanced metastatic disease in the liver, and despite its promising activity in select cancers, PD-1 blockade therapy similarly has minimal benefit in this setting. Curaxin, CBL0137, is an experimental anti-cancer drug that disrupts the binding of DNA to histones, destabilizes chromatin, and induces Z-DNA formation which may stimulate anti-tumor immune responses.
Methods: Murine cell lines of colon (CT26) and breast (4T1) cancer were interrogated for survival and CBL0137-associated DNA changes in vitro. Immunocompetent models of liver metastases followed by CBL0137 hepatic arterial infusion (HAI) were used to examine in vivo tumor cell DNA alterations, treatment responses, and the immune contexture associated with CBL0137, both alone and in combination with anti-PD-1 therapy.
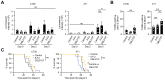
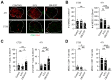
Results: CBL0137 induced immediate changes to favor tumor cell death in vitro and in vivo with an efficient tumor uptake via the HAI route. Toxicity to CBL0137 was minimal and anti-tumor treatment effects were more efficient with HAI compared to intravenous delivery. Immune effects were pronounced with CBL0137 HAI with concurrent depletion of a specific population of myeloid-derived suppressor cells and maintenance of effector T cell populations.
Conclusions: Combination of CBL0137 HAI with PD-1 blockade improved survival in 4T1 tumors but not in CT26 tumors, and therapeutic efficacy relies on the finding of simultaneous and targeted depletion of myeloid-derived suppressor cells and skewing of T cell populations to produce synergy with PD-1 blockade therapy.
Keywords: Z-DNA; hepatic arterial infusion (HAI); immunotherapy; liver tumor; myeloid-derived suppressor cells (MDSCs).
Conflict of interest statement
The authors report no competing interests.
Figures








Similar articles
-
Targeting FAcilitates Chromatin Transcription complex inhibits pleural mesothelioma and enhances immunotherapy.J Exp Clin Cancer Res. 2023 Nov 16;42(1):304. doi: 10.1186/s13046-023-02889-6. J Exp Clin Cancer Res. 2023. PMID: 37974213 Free PMC article.
-
Inflammatory cell death PANoptosis is induced by the anti-cancer curaxin CBL0137 via eliciting the assembly of ZBP1-associated PANoptosome.Inflamm Res. 2024 Apr;73(4):597-617. doi: 10.1007/s00011-024-01858-9. Epub 2024 Feb 14. Inflamm Res. 2024. PMID: 38353723
-
Targeting Features of Curaxin CBL0137 on Hematological Malignancies In Vitro and In Vivo.Biomedicines. 2023 Jan 16;11(1):230. doi: 10.3390/biomedicines11010230. Biomedicines. 2023. PMID: 36672738 Free PMC article.
-
Curaxin CBL0137 Exerts Anticancer Activity via Diverse Mechanisms.Front Oncol. 2018 Dec 7;8:598. doi: 10.3389/fonc.2018.00598. eCollection 2018. Front Oncol. 2018. PMID: 30581774 Free PMC article. Review.
-
Treatment Options in Colorectal Liver Metastases: Hepatic Arterial Infusion.Visc Med. 2017 Mar;33(1):47-53. doi: 10.1159/000454693. Epub 2017 Feb 3. Visc Med. 2017. PMID: 28612017 Free PMC article. Review.
References
-
- Abdalla E.K., Vauthey J.N., Ellis L.M., Ellis V., Pollock R., Broglio K.R., Hess K., Curley S.A. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–817. - DOI - PMC - PubMed
-
- Jerraya H., Saidani A., Khalfallah M., Bouasker I., Nouira R., Dziri C. Management of liver metastases from gastric carcinoma: Where is the evidence? Tunis Med. 2013;91:1–5. - PubMed
-
- Ursaru M., Jari I., Negru D., Scripcariu V. Local and distant recurrences—A comparative study on conservative and radical surgery for breast cancer. Chirurgia. 2015;110:38–42. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

