Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation
- PMID: 39518344
- PMCID: PMC11544904
- DOI: 10.3390/diagnostics14212376
Identification and Functional Investigation of SOX4 as a Novel Gene Underpinning Familial Atrial Fibrillation
Abstract
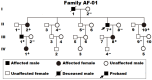
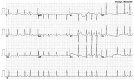
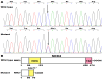
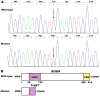
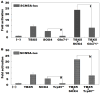
Background: Atrial fibrillation (AF) signifies the most prevalent supraventricular arrhythmia in humans and may lead to cerebral stroke, cardiac failure, and even premature demise. Aggregating strong evidence points to genetic components as a cornerstone in the etiopathogenesis of familial AF. However, the genetic determinants for AF in most patients remain elusive. Methods: A 4-generation pedigree with idiopathic AF and another cohort of 196 unrelated patients with idiopathic AF as well as 278 unrelated healthy volunteers were recruited from the Chinese population of Han ethnicity. A family-based whole-exome sequencing examination followed by a Sanger sequencing assay in all research subjects was implemented. The functional impacts of the identified SOX4 mutations were explored via a dual-reporter assay. Results: Two new heterozygous SOX4 mutations, NM_003107.3: c.211C>T; p.(Gln71*) and NM_003107.3: c.290G>A; p.(Trp97*), were observed in the family and 1 of 196 patients with idiopathic AF, respectively. The two mutations were absent in the 278 control individuals. The biochemical measurements revealed that both Gln71*- and Trp97*-mutant SOX4 failed to transactivate GJA1 (Cx43). Moreover, the two mutations nullified the synergistic activation of SCN5A by SOX4 and TBX5. Conclusions: The findings first indicate SOX4 as a gene predisposing to AF, providing a novel target for antenatal genetic screening, individualized prophylaxis, and precision treatment of AF.
Keywords: SOX4; atrial fibrillation; biochemical assay; human genetics; sequencing examination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Identification and Functional Characterization of a Novel SOX4 Mutation Predisposing to Coffin-Siris Syndromic Congenital Heart Disease.Children (Basel). 2025 May 7;12(5):608. doi: 10.3390/children12050608. Children (Basel). 2025. PMID: 40426787 Free PMC article.
-
ISL1 loss-of-function variation causes familial atrial fibrillation.Eur J Med Genet. 2020 Nov;63(11):104029. doi: 10.1016/j.ejmg.2020.104029. Epub 2020 Aug 6. Eur J Med Genet. 2020. PMID: 32771629
-
PRRX1 Loss-of-Function Mutations Underlying Familial Atrial Fibrillation.J Am Heart Assoc. 2021 Dec 7;10(23):e023517. doi: 10.1161/JAHA.121.023517. Epub 2021 Nov 30. J Am Heart Assoc. 2021. PMID: 34845933 Free PMC article.
-
Genetics of atrial fibrillation-an update of recent findings.Mol Biol Rep. 2022 Aug;49(8):8121-8129. doi: 10.1007/s11033-022-07420-2. Epub 2022 May 19. Mol Biol Rep. 2022. PMID: 35587846 Review.
-
Atrial Fibrillation: Focus on Myocardial Connexins and Gap Junctions.Biology (Basel). 2022 Mar 23;11(4):489. doi: 10.3390/biology11040489. Biology (Basel). 2022. PMID: 35453689 Free PMC article. Review.
Cited by
-
Identification and Functional Characterization of a Novel SOX4 Mutation Predisposing to Coffin-Siris Syndromic Congenital Heart Disease.Children (Basel). 2025 May 7;12(5):608. doi: 10.3390/children12050608. Children (Basel). 2025. PMID: 40426787 Free PMC article.
References
-
- Joglar J.A., Chung M.K., Armbruster A.L., Benjamin E.J., Chyou J.Y., Cronin E.M., Deswal A., Eckhardt L.L., Goldberger Z.D., Gopinathannair R., et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1–e156. doi: 10.1161/CIR.0000000000001193. - DOI - PMC - PubMed
-
- January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C., Jr., Conti J.B., Ellinor P.T., Ezekowitz M.D., Field M.E., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041. - DOI - PMC - PubMed
-
- Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. - PubMed
-
- Imberti J.F., Bonini N., Tosetti A., Mei D.A., Gerra L., Malavasi V.L., Mazza A., Lip G.Y.H., Boriani G. Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation. Biology. 2022;11:443. doi: 10.3390/biology11030443. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

