Chemotherapy Induced Corneal Changes Assessed by Corneal Confocal Microscopy: A Review
- PMID: 39518366
- PMCID: PMC11545185
- DOI: 10.3390/diagnostics14212399
Chemotherapy Induced Corneal Changes Assessed by Corneal Confocal Microscopy: A Review
Abstract
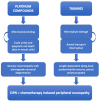
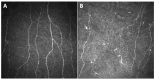
The eye, and the cornea in particular, is a common site of chemotherapy induced toxicity, and ocular side effects of both traditional and novel agents have been reported. Corneal confocal microscopy (CCM) is an in vivo technique that allows for the study of all the corneal layers in an easy, non-invasive and reproducible way via the direct visualization of corneal cell morphologies as well as of sub-basal nerve plexus. Thus, it represents a useful way to identify and monitor chemotherapy induced corneal alterations. This work aims to review the use of CCM in identifying corneal toxicity secondary to chemotherapy treatment, as regards both corneal nerves alterations in the setting of chemotherapy induced peripheral neuropathy (CIPN) and other corneal structure changes, particularly involving the corneal epithelium.
Keywords: chemotherapy; cornea; corneal confocal microscopy; corneal epithelium; corneal toxicity; peripheral neuropathy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Kunkler A.L., Binkley E.M., Mantopoulos D., Hendershot A.J., Ohr M.P., Kendra K.L., Davidorf F.H., Cebulla C.M. Known and Novel Ocular Toxicities of Biologics, Targeted Agents, and Traditional Chemotherapeutics. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019;257:1771–1781. doi: 10.1007/s00417-019-04337-8. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

