Diagnostic Performance of Faecal Immunochemical Testing (FIT) in Patients with Lynch Syndrome Scheduled for Colonoscopic Surveillance
- PMID: 39518398
- PMCID: PMC11545718
- DOI: 10.3390/diagnostics14212431
Diagnostic Performance of Faecal Immunochemical Testing (FIT) in Patients with Lynch Syndrome Scheduled for Colonoscopic Surveillance
Abstract
Background and aims: Lynch syndrome (LS) carries a substantial lifetime risk of colorectal cancer which is currently mitigated by biennial colonoscopy surveillance. Paramount to the surveillance programme is the removal of adenomas before malignant transformation but there is an associated service burden and morbidity of repeated endoscopy. We investigated if faecal immunochemical testing (FIT) for faecal haemoglobin has the diagnostic performance to replace colonoscopy.
Methods: In this retrospective cohort study, patients due to undergo planned surveillance for LS between November 2020 and April 2022 were sent two FIT kits prior to colonoscopy. Test diagnostic performance of colorectal cancer (CRC), advanced and non-advanced adenoma detection was calculated for single and double FIT strategies. A faecal-Hb of 10 µg Hb/g was considered positive.
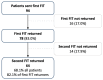
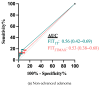
Results: In total, 78 patients, with 45 (57.7%) female, median age 52 years (IQR 41-63), completed at least one FIT and colonoscopy. The median time from FIT to colonoscopy was 47 days. A single FIT was positive in 7/30 cases of adenoma (2/3 advanced, 5/27 non-advanced). A total of 64 (82.1% of FIT1T returners) completed a second FIT. Using the greatest of the two FITs (FIT2TMAX) 8/26 (2/3 advanced, 4/23 non-advanced), patients with adenomas were identified. There were no cases of CRC. The sensitivity for adenoma detection was 23.3% and 23.1%, respectively.
Conclusions: In patients with LS awaiting colonoscopy, FIT has a low sensitivity for detecting adenomas and advanced adenomas. This is not improved by the addition of a second FIT test.
Keywords: FIT; HNPCC; LS; Lynch syndrome; faecal immunochemical testing.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

References
-
- Baglietto L., Lindor N.M., Dowty J.G., White D.M., Wagner A., Gomez Garcia E.B., Vriends A.H.J.T., Cartwright N.R., Barnetson R.A., Farrington S.M., et al. Risks of Lynch Syndrome Cancers for MSH6 Mutation Carriers. JNCI J. Natl. Cancer Inst. 2010;102:193–201. doi: 10.1093/jnci/djp473. - DOI - PMC - PubMed
-
- Monahan K.J., Bradshaw N., Dolwani S., Desouza B., Dunlop M.G., East J.E., Ilyas M., Kaur A., Lalloo F., Latchford A., et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) Gut. 2020;69:411–444. doi: 10.1136/gutjnl-2019-319915. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

