Effect of Statins and Renin-Angiotensin-Aldosterone System Inhibitors on IL-6 Levels in COVID-19 Patients
- PMID: 39518552
- PMCID: PMC11546362
- DOI: 10.3390/jcm13216414
Effect of Statins and Renin-Angiotensin-Aldosterone System Inhibitors on IL-6 Levels in COVID-19 Patients
Abstract
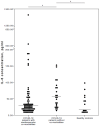
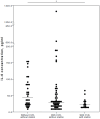
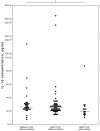
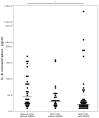
Background/Objectives: Severe clinical course and mortality from COVID-19 are mostly associated with increased concentrations of IL-6 and IL-10. Findings from clinical trials suggest that both statins and renin-angiotensin-aldosterone system inhibitors (RAASI) might have the potential to reduce unfavorable outcomes in patients with COVID-19. The aim of this study was to evaluate the effect of statins and RAASI on the cytokine concentrations in COVID-19 patients. Methods: SARS-CoV-2 infected patients were enrolled in this study, and demographic, clinical, and routine laboratory data were evaluated. Plasma cytokine levels were measured by multiplex assay. Results: COVID-19 patients with chronic cardiovascular diseases (CVD) had significantly lower median plasma IL-6 levels than COVID-19 patients with no co-morbidities (26 vs. 53 pg/mL, p = 0.021). COVID-19 patients with CVD who were taking statins had significantly lower median concentrations of IL-6 (21 vs. 44 pg/mL, p = 0.027), TNFα (21 vs. 39.5 pg/mL, p = 0.036), and IL-10 (19 vs. 25.5 pg/mL, p = 0.025) compared to COVID-19 patients with no CVD. In a binary logistic regression model, IL-6 was a significant variable, with an odds ratio value of 0.961 (95% CI 0.929-0.995). Regarding RAASI, only plasma IL-6 (22 vs. 44 pg/mL, p = 0.012) levels were found to be significantly lower in COVID-19 patients with CVD consuming these medications compared to patients who did not have any CVD. Conclusions: COVID-19 patients who had chronic cardiovascular co-morbidities and who were administered statins or RAASI had significantly lower concentrations of IL-6 than COVID-19 patients who did not have any co-morbidities. These findings suggest that the use of statins or RAASI may be of value in COVID-19 patients.
Keywords: COVID-19; IL-6; SARS-CoV-2; cardiovascular co-morbidities; medication; renin–angiotensin–aldosterone system inhibitors; statins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Udomsinprasert W., Jittikoon J., Sangroongruangsri S., Chaikledkaew U. Circulating Levels of Interleukin-6 and Interleukin-10, but Not Tumor Necrosis Factor-Alpha, as Potential Biomarkers of Severity and Mortality for COVID-19: Systematic Review with Meta-analysis. J. Clin. Immunol. 2021;41:11–22. doi: 10.1007/s10875-020-00899-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

