A Moving Target: Studying the Effect of Continuous Transcutaneous CO2 Monitoring in ELBW Infants During an Equipoise Shift
- PMID: 39518610
- PMCID: PMC11547053
- DOI: 10.3390/jcm13216472
A Moving Target: Studying the Effect of Continuous Transcutaneous CO2 Monitoring in ELBW Infants During an Equipoise Shift
Abstract
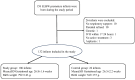
Objectives: To assess whether continuous non-invasive pCO2 monitoring by transcutaneous pCO2 monitor (TCpCO2) among extremely low birth weight (ELBW) premature infants, during the first week of life, will decrease the rate of high-grade intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL) or the combined outcome of IVH/PVL and death. Methods: This was a prospective, observational, multicenter study. Due to ethical constraints, allocation was based on TCpCO2 monitor availability. ELBW infants were either monitored by TCpCO2 monitor (Sentec, Therwil, Switzerland) (study group), or recruited to the control group if a TCpCO2 monitor was not available. Results: A total of 132 ELBW infants participated in the study. The size of the study group (106 infants) and the control group (26 infants) differed because monitor availability increased during the study period reflecting change in standard of care. The groups had comparable gestational age and baseline characteristics. No difference was found in the rate of IVH/PVL in the study vs. control groups (10% vs. 4%; p = 0.7, respectively), or in the combined outcome of PVL/IVH and death (16% vs. 15%; p = 1.0, respectively). Conclusions: This study demonstrates the challenges in conducting a prospective controlled trial in a rapidly evolving medical field. While the study began with a clear equipoise, this balance shifted as the care team gained more experience with TCpCO2 monitoring among the study population, despite the absence of new clinical evidence to justify such a shift. Consequently, the small control group limited our ability to draw definitive conclusions regarding the study's objective. However, our findings may increase awareness of continuous non-invasive pCO2 monitoring in extremely premature infants.
Keywords: intraventricular hemorrhage; non-invasive CO2 monitoring; premature infant; transcutaneous CO2 monitoring.
Conflict of interest statement
The authors declare no conflicts of interest. Sentec had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
-
- Fabres J., Carlo W.A., Phillips V., Howard G., Ambalavanan N. Both extremes of arterial carbon dioxide pressure and the magnitude of fluctuations in arterial carbon dioxide pressure are associated with severe intraventricular hemorrhage in preterm infants. Pediatrics. 2007;119:299–305. doi: 10.1542/peds.2006-2434. - DOI - PubMed
LinkOut - more resources
Full Text Sources