NF-κB in Alzheimer's Disease: Friend or Foe? Opposite Functions in Neurons and Glial Cells
- PMID: 39518906
- PMCID: PMC11545113
- DOI: 10.3390/ijms252111353
NF-κB in Alzheimer's Disease: Friend or Foe? Opposite Functions in Neurons and Glial Cells
Abstract
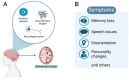
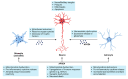
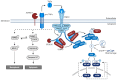
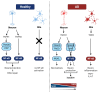
Alzheimer's disease (AD) is a devasting neurodegenerative disease afflicting mainly glutamatergic neurons together with a massive neuroinflammation mediated by the transcription factor NF-κB. A 65%-plus increase in Alzheimer's patients by 2050 might be a major threat to society. Hallmarks of AD are neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau and amyloid beta (Aβ) plaques. Here, we review the potential involvement of transcription factor NF-κB by hereditary mutations of the tumor necrosis factor pathway in AD patients. One of the greatest genetic risk factors is APOE4. Recently, it was shown that the APOE4 allele functions as a null allele in human astrocytes not repressing NF-κB anymore. Moreover, NF-κB seems to be involved in the repair of DNA double-strand breaks during healthy learning and memory, a function blunted in AD. NF-κB could be a friend to healthy neurons by repressing apoptosis and necroptosis. But a loss of neuronal NF-κB and activation of glial NF-κB in AD makes it a foe of neuronal survival. Hopeful therapies include TNFR2 receptor bodies relieving the activation of glial NF-κB by TNFα.
Keywords: APOE4; Alzheimer’s disease; DNA breaks; DNA damage repair; NF-κB; TNF; glial cells; neuroinflammation; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Alzheimer A. über eigenartige Krankheitsfälle des späteren Alters. Z Für Gesamte Neurol. Psychiatr. 1911;4:356–385. doi: 10.1007/BF02866241. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

