Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration
- PMID: 39518910
- PMCID: PMC11545389
- DOI: 10.3390/ijms252111357
Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration
Abstract
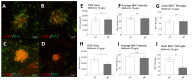
Neovascular age-related macular degeneration (nAMD), a leading cause of blindness in older adults, presents a challenging pathophysiology involving choroidal neovascularization (CNV) and retinal degeneration. Current treatments relying on intravitreal (IVT) administration of anti-angiogenic agents are costly and of moderate effectiveness. Metformin, the common anti-diabetic drug, has been associated with decreased odds of developing AMD. Studies have shown that metformin can mitigate cellular aging, neoangiogenesis, and inflammation across multiple diseases. This preclinical study assessed metformin's impact on vessel growth using choroidal explants before exploring IVT metformin's effects on laser-induced CNV and light-induced retinal degeneration in C57BL/6J and BALB/cJ mice, respectively. Metformin reduced new vessel growth in choroidal explants in a dose-dependent relationship. Following laser induction, IVT metformin suppressed CNV and decreased peripheral infiltration of IBA1+ macrophages/microglia. Furthermore, IVT metformin protected against retinal thinning in response to light-induced degeneration. IVT metformin downregulated genes in the choroid and retinal pigment epithelium which are associated with angiogenesis and inflammation, two key processes that drive nAMD progression. These findings underscore metformin's capacity as an anti-angiogenic and neuroprotective agent, demonstrating this drug's potential as an accessible option to help manage nAMD.
Keywords: age-related macular degeneration; choroidal neovascularization; intravitreal injection; metformin; retinal degeneration.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures




Similar articles
-
Granzyme B degrades extracellular matrix and promotes inflammation and choroidal neovascularization.Angiogenesis. 2024 Aug;27(3):351-373. doi: 10.1007/s10456-024-09909-9. Epub 2024 Mar 18. Angiogenesis. 2024. PMID: 38498232 Free PMC article.
-
Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model.Exp Eye Res. 2021 Feb;203:108391. doi: 10.1016/j.exer.2020.108391. Epub 2020 Dec 8. Exp Eye Res. 2021. PMID: 33307075
-
Inhibition of APE1/Ref-1 redox activity rescues human retinal pigment epithelial cells from oxidative stress and reduces choroidal neovascularization.Redox Biol. 2014 Feb 21;2:485-94. doi: 10.1016/j.redox.2014.01.023. eCollection 2014. Redox Biol. 2014. PMID: 24624338 Free PMC article.
-
Clinical evidence of intravitreal triamcinolone acetonide in the management of age-related macular degeneration.Curr Drug Targets. 2011 Feb;12(2):149-72. doi: 10.2174/138945011794182746. Curr Drug Targets. 2011. PMID: 20887246 Review.
-
Light as a Mediator of Acute and Chronic Retina Degeneration.Adv Exp Med Biol. 2025;1468:247-251. doi: 10.1007/978-3-031-76550-6_41. Adv Exp Med Biol. 2025. PMID: 39930204 Review.
Cited by
-
The Anti-Aging Mechanism of Metformin: From Molecular Insights to Clinical Applications.Molecules. 2025 Feb 10;30(4):816. doi: 10.3390/molecules30040816. Molecules. 2025. PMID: 40005128 Free PMC article. Review.
-
Exploring the Protective Effects of Traditional Antidiabetic Medications and Novel Antihyperglycemic Agents in Diabetic Rodent Models.Pharmaceuticals (Basel). 2025 May 1;18(5):670. doi: 10.3390/ph18050670. Pharmaceuticals (Basel). 2025. PMID: 40430489 Free PMC article. Review.
References
-
- GBD 2019 Blindness and Vision Impairment Collaborators. Vision Loss Expert Group of the Global Burden of Disease Study Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–e160. doi: 10.1016/S2214-109X(20)30489-7. - DOI - PMC - PubMed
-
- Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- R01 EY034486/EY/NEI NIH HHS/United States
- M2018042/BrightFocus Foundation
- NA/Thome Memorial Foundation Award
- NA/Institute for Translational Medicine
- K08 EY030923/NH/NIH HHS/United States
- NA/The University of Chicago Women's Board
- FP067271-01-PR/Illinois Society for the Prevention of Blindness
- R01 EY034486/NH/NIH HHS/United States
- NA/FORE-I Foundation
- NA/Research to Prevent Blindness Sybil B. Harrington Career Development Award for Macular Degeneration
- K08 EY030923/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources

