Noradrenaline Protects Human Microglial Cells (HMC3) Against Apoptosis and DNA Damage Induced by LPS and Aβ1-42 Aggregates In Vitro
- PMID: 39518952
- PMCID: PMC11546654
- DOI: 10.3390/ijms252111399
Noradrenaline Protects Human Microglial Cells (HMC3) Against Apoptosis and DNA Damage Induced by LPS and Aβ1-42 Aggregates In Vitro
Abstract
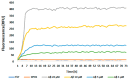
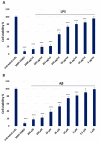
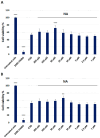
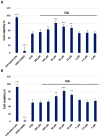
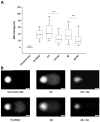
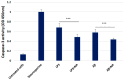
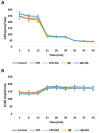
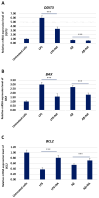
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder, characterized by the accumulation of amyloid-beta (Aβ) plaques and neuroinflammation. This study investigates the protective effects of noradrenaline (NA) on human microglial cells exposed to lipopolysaccharides (LPS) and Aβ aggregates-major contributors to inflammation and cellular damage in AD. The reduced Aβ aggregation in the HMC3 human microglial cells co-treated with Aβ and NA was confirmed by thioflavin T (ThT) assay, fluorescent ThT staining, and immunocytochemistry (ICC). The significantly increased viability of HMC3 cells after 48 h of incubation with NA at 50 µM, 25 µM, and 10 µM, exposed to IC50 LPS and IC50 Aβ, was confirmed by XTT and LDH assays. Moreover, we found that NA treatment at 25 μM and 50 μM concentrations in HMC3 cells exposed to IC50 LPS or IC50 Aβ results in an increased proliferation of HMC3 cells, their return to normal morphology, decreased levels of DNA damage, reduced caspase-3 activity, decreased expression of pro-apoptotic DDIT3 and BAX, and increased expression of anti-apoptotic BCL-2 genes and proteins, leading to enhanced cell survival, when compared to that of the HMC3 cells treated only with IC50 LPS or IC50 Aβ. Furthermore, we showed that NA induces the degradation of both extracellular and intracellular Aβ deposits and downregulates hypoxia-inducible factor 1α (HIF-1α), which is linked to impaired Aβ clearance and AD progression. These findings indicate that NA holds promise as a therapeutic target to address microglial dysfunction and potentially slow the progression of AD. Its neuroprotective effects, particularly in reducing inflammation and regulating microglial activity, warrant further investigation into its broader role in mitigating neuroinflammation and preserving microglial function in AD.
Keywords: Alzheimer’s disease; Bax; Bcl-2; HIF-1α; LPS; amyloid beta; caspase-3; microglia; neuroinflammation; noradrenaline.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

