Murine Regulatory CD4+ T Cells Are Increased in Leukemic Spleens and Show High Co-Expression of Co-Regulatory Molecules CD39, CD73, PD1, and TIGIT
- PMID: 39518969
- PMCID: PMC11546357
- DOI: 10.3390/ijms252111412
Murine Regulatory CD4+ T Cells Are Increased in Leukemic Spleens and Show High Co-Expression of Co-Regulatory Molecules CD39, CD73, PD1, and TIGIT
Abstract
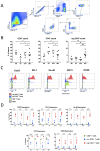
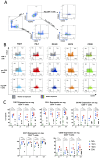
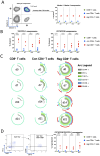
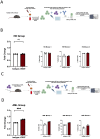
Comprehensive characterization of AML-associated T cells during disease progression is essential to identify relevant immune escape mechanisms and new immunotherapeutic approaches. Investigating the processes that lead to an immunosuppressive environment under progression of AML is difficult in humans, because by the time of diagnosis the disease is often progressed far beyond the initial stages. Therefore, to investigate T-cell phenotypes during progression a C57BL/6 mouse model was used. The CD3+ T cells were characterized by performing multiparametric flow analyses at different time points (day 0 = healthy mice, day 7, day 14, and day 21). The study revealed that the spleen is highly infiltrated by reg CD4+ T cells at day 21 of AML progression. These spleen-infiltrating reg CD4+ T cells mainly showed an effector memory differentiation with high expression and co-expression of the checkpoint molecules TIGIT, PD-1, OX40, and the two ectoenzymes CD39 and CD73. Substantial expression of the checkpoint molecules was restricted to the central memory and effector memory compartments. Furthermore, functional evaluation of TIGIT was performed. Blocking TIGIT resulted in a significantly increased lysis of C1498 AML cells in cocultures with AML-primed CD3+ T cells. Together these data confirm that the expression of the checkpoint receptor TIGIT is relevant for dysfunction of AML-associated T cells and, thus, represents a suitable target for future immunotherapeutic approaches.
Keywords: CD39; CD73; PD-1; T cells; TIGIT; antitumor immunity; checkpoint molecules; murine AML; regulatory CD4+ T cells; spleen.
Conflict of interest statement
F.B.: Travel grant, Daiichi Sankyo, Servier, Novartis; advisory board by Jazz. GmbH, Daiichi Sankyo. W.F.: personal fees and nonfinancial support from AbbVie; grants, personal fees, and nonfinancial support from Amgen and Pfizer; and personal fees from Jazz Pharmaceuticals, Celgene, Morphosys, Ariad/Incyte, stem line therapeutics Daiichi Sankyo, and Servier outside the submitted work; in addition, W.F. has a patent for Amgen issued; support for medical writing: Amgen, Pfizer, and AbbVie. J.W.: patent for Amgen issued. The remaining authors declare that they have no conflict of interest. C.B.: Invited speaker for AOK Germany, Astra Zeneca, Merck Serono, med update. and Roche Pharma; lectures for Sanofi Aventis; advisory board: Astra Zeneca, AOK Germany, Bayer Healthcare, BioNTech, Lindis Biotech, Merck Serono, and Sanofi Aventis; advisory role, Board of DGHO Advisors: German Society of Hematology and Oncology (DGHO); leadership role: Hamburg Cancer Society (HKG), National Network of German Cancer Centers (CCC)/German Cancer Aid (DKH); advisory role, Board of Directors Oncology Center Certification Committee: German Cancer Society (DKG); other financial or nonfinancial interests: more than 95 clinical trials, local PI, institutional, and of nonfinancial interest, and our department is involved in several clinical trials sponsored by industry and cooperative groups at which we hold participant roles and local PI roles and PI roles.
Figures
References
-
- Daver N.G., Iqbal S., Renard C., Chan R.J., Hasegawa K., Hu H., Tse P., Yan J., Zoratti M.J., Xie F., et al. Treatment Outcomes for Newly Diagnosed, Treatment-Naïve TP53-Mutated Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. J. Hematol. Oncol. 2023;16:19. doi: 10.1186/s13045-023-01417-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

