High Quantum Yields and Biomedical Fluorescent Imaging Applications of Photosensitized Trivalent Lanthanide Ion-Based Nanoparticles
- PMID: 39518971
- PMCID: PMC11546352
- DOI: 10.3390/ijms252111419
High Quantum Yields and Biomedical Fluorescent Imaging Applications of Photosensitized Trivalent Lanthanide Ion-Based Nanoparticles
Abstract
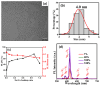
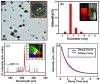
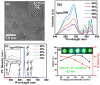
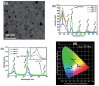
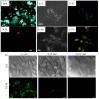
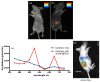
In recent years, significant advances in enhancing the quantum yield (QY) of trivalent lanthanide (Ln3+) ion-based nanoparticles have been achieved through photosensitization, using host matrices or capping organic ligands as photosensitizers to absorb incoming photons and transfer energy to the Ln3+ ions. The Ln3+ ion-based nanoparticles possess several excellent fluorescent properties, such as nearly constant transition energies, atomic-like sharp transitions, long emission lifetimes, large Stokes shifts, high photostability, and resistance to photobleaching; these properties make them more promising candidates as next-generation fluorescence probes in the visible region, compared with other traditional materials such as organic dyes and quantum dots. However, their QYs are generally low and thus need to be improved to facilitate and extend their applications. Considerable efforts have been made to improve the QYs of Ln3+ ion-based nanoparticles through photosensitization. These efforts include the doping of Ln3+ ions into host matrices or capping the nanoparticles with organic ligands. Among the Ln3+ ion-based nanoparticles investigated in previous studies, this review focuses on those containing Eu3+, Tb3+, and Dy3+ ions with red, green, and yellow emission colors, respectively. The emission intensities of Eu3+ and Tb3+ ions are stronger than those of other Ln3+ ions; therefore, the majority of the reported studies focused on Eu3+ and Tb3+ ion-based nanoparticles. This review discusses the principles of photosensitization, several examples of photosensitized Ln3+ ion-based nanoparticles, and in vitro and in vivo biomedical fluorescent imaging (FI) applications. This information provides valuable insight into the development of Ln3+ ion-based nanoparticles with high QYs through photosensitization, with future potential applications in biomedical FI.
Keywords: Ln3+ ion-based nanoparticle; biomedical fluorescent imaging application; high quantum yield; host matrix; organic ligand; photosensitization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












Similar articles
-
High-Quantum-Yield Ultrasmall Ln2O3 (Ln = Eu, Tb, or Dy) Nanoparticle Colloids in Aqueous Media Obtained via Photosensitization.Langmuir. 2023 Oct 31;39(43):15338-15342. doi: 10.1021/acs.langmuir.3c02229. Epub 2023 Oct 19. Langmuir. 2023. PMID: 37856331
-
Ga(3+)/Ln(3+) Metallacrowns: A Promising Family of Highly Luminescent Lanthanide Complexes That Covers Visible and Near-Infrared Domains.J Am Chem Soc. 2016 Apr 20;138(15):5100-9. doi: 10.1021/jacs.6b00984. Epub 2016 Apr 8. J Am Chem Soc. 2016. PMID: 27015360
-
Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights.Acc Chem Res. 2018 Jun 19;51(6):1404-1414. doi: 10.1021/acs.accounts.8b00060. Epub 2018 May 7. Acc Chem Res. 2018. PMID: 29733571
-
A critical comparison of lanthanide based upconversion nanoparticles to fluorescent proteins, semiconductor quantum dots, and carbon dots for use in optical sensing and imaging.Methods Appl Fluoresc. 2019 Mar 27;7(2):022002. doi: 10.1088/2050-6120/ab0bfa. Methods Appl Fluoresc. 2019. PMID: 30822759 Review.
-
Recent advances in synthesis and surface modification of lanthanide-doped upconversion nanoparticles for biomedical applications.Biotechnol Adv. 2012 Nov-Dec;30(6):1551-61. doi: 10.1016/j.biotechadv.2012.04.009. Epub 2012 Apr 27. Biotechnol Adv. 2012. PMID: 22561011 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

