Development and Characterization of a Human Mammary Epithelial Cell Culture Model for the Blood-Milk Barrier-A Contribution from the ConcePTION Project
- PMID: 39519007
- PMCID: PMC11546117
- DOI: 10.3390/ijms252111454
Development and Characterization of a Human Mammary Epithelial Cell Culture Model for the Blood-Milk Barrier-A Contribution from the ConcePTION Project
Abstract
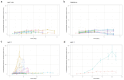
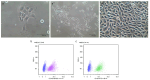
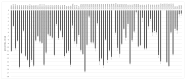
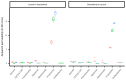
It is currently impossible to perform an evidence-based risk assessment for medication use during breastfeeding. The ConcePTION project aims to provide information about the use of medicines during lactation. The study aimed to develop and characterize an in vitro model of the blood-milk barrier to determine the extent of the milk transfer of xenobiotics, relying on either on human mammary epithelial cells (hMECs) or immortalized cell lines derived from breast tissue. The hMECs were cultured and characterized for epithelial markers; further, the ability to form an epithelial barrier was investigated. Drug transporter functionality in the cultured hMECs was analyzed with specific probe substrates. The hMECs showed an epithelial morphology and the expression of epithelial markers and tight junctions. They formed a reproducible tight barrier with a transepithelial electrical resistance greater than 400 Ωcm2, unlike immortalized cell lines. Different levels of mRNA expression were detected for 81 genes of membrane transporters. Functional assays showed no evidence for the transporter-mediated secretion of medicines across the hMECs. Nevertheless, the hMEC-based in vitro model covered a 50-fold range of permeability values, differentiating between passive transcellular and paracellular-mediated transport. The cultured hMECs proved to be a promising in vitro model for biorelevance; the wide characterization of hMECs makes them useful for studying medicine partitioning in milk.
Keywords: blood–milk barrier; breastfeeding; cell membrane permeability; human mammary epithelium; in vitro barrier model; membrane transport proteins; primary cell culture; transepithelial electrical resistance.
Conflict of interest statement
All authors have read the journal’s authorship agreement and policy on the disclosure of potential conflicts of interest. Pieter Annaert is co-owner of BioNotus GCV. The research project leading to these results was conducted as part of the ConcePTION consortium. This manuscript only reflects the personal views of the stated authors. The company had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

