Double Heterozygous Pathogenic Variants in TP53 and CHEK2 in Boy with Undifferentiated Embryonal Sarcoma of the Liver
- PMID: 39519042
- PMCID: PMC11545958
- DOI: 10.3390/ijms252111489
Double Heterozygous Pathogenic Variants in TP53 and CHEK2 in Boy with Undifferentiated Embryonal Sarcoma of the Liver
Abstract
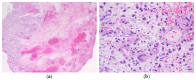
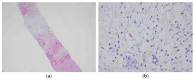
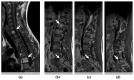
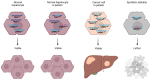
Undifferentiated embryonal sarcoma of the liver is a rare mesenchymal malignancy that predominantly occurs in children. The relationship between this tumor entity and germline pathogenic variants (PVs) remains undefined. Here, we present the clinical case of a male patient diagnosed with undifferentiated embryonal sarcoma of the liver. Both germline and tumor samples were analyzed using next-generation sequencing. In the tumor tissue, PVs in TP53 (NM_000546.5):c.532del p.(His178Thrfs*69) and CHEK2 (NM_007194.4):c.85C>T p.(Gln29*) were identified, with both confirmed to be of germline origin. Copy number analyses indicated a loss of the wildtype TP53 allele in the tumor, consistent with a second hit, while it was the variant CHEK2 allele that was lost in the tumor. Our data indicate that the germline TP53 PV acts as a driver of tumorigenesis in the reported case and support a complex interaction between the germline TP53 and CHEK2 PVs. This case highlights the dynamic interplays of genetic alterations in tumorigenesis and emphasizes the need for continued investigation into the complex interactions between TP53 and CHEK2 PVs and into the association of undifferentiated embryonal sarcoma of the liver and Li-Fraumeni syndrome.
Keywords: CHEK2; TP53; double heterozygosity; embryonal sarcoma of the liver; synthetic lethality.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
CHEK2 is not a Li-Fraumeni syndrome gene: time to update public resources.J Med Genet. 2023 Nov 27;60(12):1215-1217. doi: 10.1136/jmg-2023-109464. J Med Genet. 2023. PMID: 37536919
-
The genomic landscape of undifferentiated embryonal sarcoma of the liver is typified by C19MC structural rearrangement and overexpression combined with TP53 mutation or loss.PLoS Genet. 2020 Apr 20;16(4):e1008642. doi: 10.1371/journal.pgen.1008642. eCollection 2020 Apr. PLoS Genet. 2020. PMID: 32310940 Free PMC article.
-
A substantial proportion of apparently heterozygous TP53 pathogenic variants detected with a next-generation sequencing hereditary pan-cancer panel are acquired somatically.Hum Mutat. 2020 Jan;41(1):203-211. doi: 10.1002/humu.23910. Epub 2019 Sep 23. Hum Mutat. 2020. PMID: 31490007 Free PMC article.
-
Li-Fraumeni syndrome: not a straightforward diagnosis anymore-the interpretation of pathogenic variants of low allele frequency and the differences between germline PVs, mosaicism, and clonal hematopoiesis.Breast Cancer Res. 2019 Sep 18;21(1):107. doi: 10.1186/s13058-019-1193-1. Breast Cancer Res. 2019. PMID: 31533767 Free PMC article. Review.
-
Undifferentiated embryonal sarcoma of the liver: a concise review.Arch Pathol Lab Med. 2015 Feb;139(2):269-73. doi: 10.5858/arpa.2013-0463-RS. Arch Pathol Lab Med. 2015. PMID: 25611111 Review.
References
-
- Goudie C., Cullinan N., Villani A., Mathews N., van Engelen K., Malkin D., Irwin M.S., Foulkes W.D. Retrospective evaluation of a decision-support algorithm (MIPOGG) for genetic referrals for children with neuroblastic tumors. Pediatr. Blood Cancer. 2018;65:e27390. doi: 10.1002/pbc.27390. - DOI - PubMed
-
- Ripperger T., Bielack S.S., Borkhardt A., Brecht I.B., Burkhardt B., Calaminus G., Debatin K.M., Deubzer H., Dirksen U., Eckert C., et al. Childhood cancer predisposition syndromes-A concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am. J. Med. Genet. A. 2017;173:1017–1037. doi: 10.1002/ajmg.a.38142. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

