Advancements in LAMP-Based Diagnostics: Emerging Techniques and Applications in Viral Detection with a Focus on Herpesviruses in Transplant Patient Management
- PMID: 39519059
- PMCID: PMC11546353
- DOI: 10.3390/ijms252111506
Advancements in LAMP-Based Diagnostics: Emerging Techniques and Applications in Viral Detection with a Focus on Herpesviruses in Transplant Patient Management
Abstract
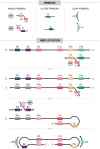
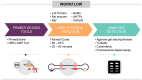
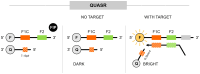
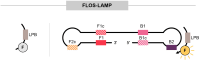
Loop-mediated isothermal amplification (LAMP) is a highly effective molecular diagnostic technique, particularly advantageous for point-of-care (POC) settings. In recent years, LAMP has expanded to include various adaptations such as DARQ-LAMP, QUASR, FLOS-LAMP, displacement probes and molecular beacons. These methods enable multiplex detection of multiple targets in a single reaction, enhancing cost-effectiveness and diagnostic efficiency. Consequently, LAMP has gained significant traction in diagnosing diverse viruses, notably during the COVID-19 pandemic. However, its application for detecting Herpesviridae remains relatively unexplored. This group of viruses is of particular interest due to their latency and potential reactivation, crucial for immunocompromised patients, including organ and hematopoietic stem cell transplant recipients. This review highlights recent advancements in LAMP for virus diagnosis and explores current research trends and future prospects, emphasizing the detection challenges posed by Herpesviridae.
Keywords: Herpesviridae; LAMP; isothermal amplification; point-of-care; viral detection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
High-Surety Isothermal Amplification and Detection of SARS-CoV-2.mSphere. 2021 May 19;6(3):e00911-20. doi: 10.1128/mSphere.00911-20. mSphere. 2021. PMID: 34011690 Free PMC article.
-
Sequence-specific and multiplex detection of COVID-19 virus (SARS-CoV-2) using proofreading enzyme-mediated probe cleavage coupled with isothermal amplification.Biosens Bioelectron. 2021 Apr 15;178:113041. doi: 10.1016/j.bios.2021.113041. Epub 2021 Jan 28. Biosens Bioelectron. 2021. PMID: 33545551 Free PMC article.
-
Loop-mediated isothermal amplification (LAMP): An effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2.Rev Med Virol. 2021 Nov;31(6):e2215. doi: 10.1002/rmv.2215. Epub 2021 Jan 21. Rev Med Virol. 2021. PMID: 33476080 Free PMC article. Review.
-
Accessible LAMP-Enabled Rapid Test (ALERT) for Detecting SARS-CoV-2.Viruses. 2021 Apr 23;13(5):742. doi: 10.3390/v13050742. Viruses. 2021. PMID: 33922716 Free PMC article.
-
LAMP Diagnostics at the Point-of-Care: Emerging Trends and Perspectives for the Developer Community.Expert Rev Mol Diagn. 2021 Jan;21(1):43-61. doi: 10.1080/14737159.2021.1873769. Epub 2021 Jan 27. Expert Rev Mol Diagn. 2021. PMID: 33474990 Review.
References
-
- Marin A.M., Zanette D.L., Nardin J.M., Munhoz E.C., Blanes L., Soligo Sanchuki H.B., Boçon de Araújo Munhoz F., de Oliveira Coelho B., Aoki M.N. Fluorescent and Colorimetric RT-LAMP as a Rapid and Specific Qualitative Method for Chronic Myeloid Leukemia Diagnosis. Anal. Biochem. 2022;641:114541. doi: 10.1016/j.ab.2021.114541. - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

