Diverse Roles of the LINC Complex in Cellular Function and Disease in the Nervous System
- PMID: 39519078
- PMCID: PMC11545860
- DOI: 10.3390/ijms252111525
Diverse Roles of the LINC Complex in Cellular Function and Disease in the Nervous System
Abstract
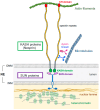
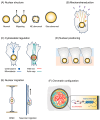
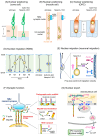
The linker of nucleoskeleton and cytoskeleton (LINC) complex, which spans the nuclear envelope, physically connects nuclear components to the cytoskeleton and plays a pivotal role in various cellular processes, including nuclear positioning, cell migration, and chromosomal configuration. Studies have revealed that the LINC complex is essential for different aspects of the nervous system, particularly during development. The significance of the LINC complex in neural lineage cells is further corroborated by the fact that mutations in genes associated with the LINC complex have been implicated in several neurological diseases, including neurodegenerative and psychiatric disorders. In this review, we aimed to summarize the expanding knowledge of LINC complex-related neuronal functions and associated neurological diseases.
Keywords: LINC complex; cytoskeleton; diseases; nervous system; nuclear envelope.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
LINC complex proteins in development and disease.Curr Top Dev Biol. 2014;109:287-321. doi: 10.1016/B978-0-12-397920-9.00004-4. Curr Top Dev Biol. 2014. PMID: 24947240 Review.
-
Integrity of the Linker of Nucleoskeleton and Cytoskeleton Is Required for Efficient Herpesvirus Nuclear Egress.J Virol. 2017 Sep 12;91(19):e00330-17. doi: 10.1128/JVI.00330-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724767 Free PMC article.
-
LINCing complex functions at the nuclear envelope: what the molecular architecture of the LINC complex can reveal about its function.Nucleus. 2013 Jan-Feb;4(1):29-36. doi: 10.4161/nucl.23387. Epub 2013 Jan 1. Nucleus. 2013. PMID: 23324460 Free PMC article.
-
Life outside the LINC complex - Do SUN proteins have LINC-independent functions?Bioessays. 2024 Aug;46(8):e2400034. doi: 10.1002/bies.202400034. Epub 2024 May 27. Bioessays. 2024. PMID: 38798157 Free PMC article. Review.
-
Dynamic regulation of LINC complex composition and function across tissues and contexts.FEBS Lett. 2023 Nov;597(22):2823-2832. doi: 10.1002/1873-3468.14757. Epub 2023 Oct 24. FEBS Lett. 2023. PMID: 37846646 Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

