Gestational Diabetes-like Fuels Impair Mitochondrial Function and Long-Chain Fatty Acid Uptake in Human Trophoblasts
- PMID: 39519087
- PMCID: PMC11546831
- DOI: 10.3390/ijms252111534
Gestational Diabetes-like Fuels Impair Mitochondrial Function and Long-Chain Fatty Acid Uptake in Human Trophoblasts
Abstract
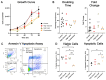
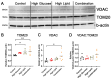
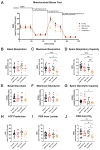
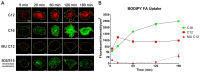
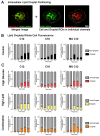
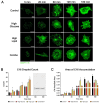
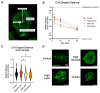
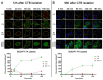
In the parent, gestational diabetes mellitus (GDM) causes both hyperglycemia and hyperlipidemia. Despite excess lipid availability, infants exposed to GDM are at risk for essential long-chain polyunsaturated fatty acid (LCPUFA) deficiency. Isotope studies have confirmed less LCPUFA transfer from the parent to the fetus, but how diabetic fuels impact placental fatty acid (FA) uptake and lipid droplet partitioning is not well-understood. We evaluated the effects of high glucose conditions, high lipid conditions, and their combination on trophoblast growth, viability, mitochondrial bioenergetics, BODIPY-labeled fatty acid (FA) uptake, and lipid droplet dynamics. The addition of four carbons or one double bond to FA acyl chains dramatically affected the uptake in both BeWo and primary isolated cytotrophoblasts (CTBs). The uptake was further impacted by media exposure. The combination-exposed trophoblasts had more mitochondrial protein (p = 0.01), but impaired maximal and spare respiratory capacities (p < 0.001 and p < 0.0001), as well as lower viability (p = 0.004), due to apoptosis. The combination-exposed trophoblasts had unimpaired uptake of BODIPY C12 but had significantly less whole-cell and lipid droplet uptake of BODIPY C16, with an altered lipid droplet count, area, and subcellular localization, whereas these differences were not seen with individual high glucose or lipid exposure. These findings bring us closer to understanding how GDM perturbs active FA transport to increase the risk of adverse outcomes from placental and neonatal lipid accumulation alongside LCPUFA deficiency.
Keywords: fatty acids; gestational diabetes mellitus; lipid droplets; mitochondria; trophoblast.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Gregory E.C.W., Ely D.M. Trends and Characteristics in Gestational Diabetes: United States, 2016–2020. Natl. Vital Stat. Rep. 2022;71:1–15. - PubMed
-
- Centers for Disease Control and Prevention About Gestational Diabetes. [(accessed on 27 June 2024)]; Available online: https://www.cdc.gov/diabetes/about/gestational-diabetes.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

