Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts
- PMID: 39519090
- PMCID: PMC11545928
- DOI: 10.3390/ijms252111538
Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts
Abstract
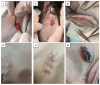
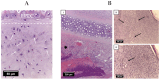
In this study, the optimal engraving parameters were determined through the analysis of scanning electron microscopy (SEM) data, as follows: a laser power density of 5.5 × 105 W/cm2, an irradiation rate of 0.1 mm/s, a well radius of 60 μm, a distance between well centers of 200 μm, and a number of passes for each well of 20. After 1 week of in vitro cultivation, chondrocytes were located on the surface of the scaffolds, in the sockets and lacunae of decellularized cartilage. When implanted into animals, both cellular and acellular scaffolds were able to support cartilage in-growth and complete regeneration of the defect without clear boundaries with normal tissue. Nevertheless, the scaffolds populated with cells exhibited superior biocompatibility and were not subject to rejection, in contrast to cell-free constructs.
Keywords: ear cartilage; laser engraving; nasal chondrocytes; regenerative medicine; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Beketov E.E., Isaeva E.V., Yakovleva N.D., Demyashkin G.A., Arguchinskaya N.V., Kisel A.A., Lagoda T.S., Malakhov E.P., Kharlov V.I., Osidak E.O., et al. Bioprinting of Cartilage with Bioink Based on High-Concentration Collagen and Chondrocytes. Int. J. Mol. Sci. 2021;22:11351. doi: 10.3390/ijms222111351. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

