New Insights into the Fanconi Anemia Pathogenesis: A Crosstalk Between Inflammation and Oxidative Stress
- PMID: 39519169
- PMCID: PMC11547024
- DOI: 10.3390/ijms252111619
New Insights into the Fanconi Anemia Pathogenesis: A Crosstalk Between Inflammation and Oxidative Stress
Abstract
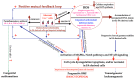
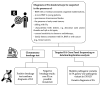
Fanconi anemia (FA) represents a rare hereditary disease; it develops due to germline pathogenic variants in any of the 22 currently discovered FANC genes, which interact with the Fanconi anemia/breast cancer-associated (FANC/BRCA) pathway to maintain genome integrity. FA is characterized by a triad of clinical traits, including congenital anomalies, bone marrow failure (BMF) and multiple cancer susceptibility. Due to the complex genetic background and a broad spectrum of FA clinical symptoms, the diagnostic process is complex and requires the use of classical cytogenetic, molecular cytogenetics and strictly molecular methods. Recent findings indicate the interplay of inflammation, oxidative stress, disrupted mitochondrial metabolism, and impaired intracellular signaling in the FA pathogenesis. Additionally, a shift in the balance towards overproduction of proinflammatory cytokines and prooxidant components in FA is associated with advanced myelosuppression and ultimately BMF. Although the mechanism of BMF is very complex and needs further clarification, it appears that mutual interaction between proinflammatory cytokines and redox imbalance causes pancytopenia. In this review, we summarize the available literature regarding the clinical phenotype, genetic background, and diagnostic procedures of FA. We also highlight the current understanding of disrupted autophagy process, proinflammatory state, impaired signaling pathways and oxidative genotoxic stress in FA pathogenesis.
Keywords: Fanconi anemia; autophagy; bone marrow failure; cancers; inflammatory process; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A new frontier in Fanconi anemia: From DNA repair to ribosome biogenesis.Blood Rev. 2022 Mar;52:100904. doi: 10.1016/j.blre.2021.100904. Epub 2021 Oct 31. Blood Rev. 2022. PMID: 34750031 Review.
-
Emerging functions of the Fanconi anemia pathway at a glance.J Cell Sci. 2017 Aug 15;130(16):2657-2662. doi: 10.1242/jcs.204909. J Cell Sci. 2017. PMID: 28811338 Free PMC article. Review.
-
[Genetic analysis of Japanese patients with Fanconi anemia: novel findings].Rinsho Ketsueki. 2019;60(6):691-701. doi: 10.11406/rinketsu.60.691. Rinsho Ketsueki. 2019. PMID: 31281162 Review. Japanese.
-
Recent discoveries in the molecular pathogenesis of the inherited bone marrow failure syndrome Fanconi anemia.Blood Rev. 2017 May;31(3):93-99. doi: 10.1016/j.blre.2016.10.002. Epub 2016 Oct 13. Blood Rev. 2017. PMID: 27760710 Free PMC article. Review.
-
Yeast two-hybrid screens imply involvement of Fanconi anemia proteins in transcription regulation, cell signaling, oxidative metabolism, and cellular transport.Exp Cell Res. 2003 Oct 1;289(2):211-21. doi: 10.1016/s0014-4827(03)00261-1. Exp Cell Res. 2003. PMID: 14499622
References
-
- Reyes P., García-de Teresa B., Juárez U., Pérez-Villatoro F., Fiesco-Roa M.O., Rodríguez A., Molina B., Villarreal-Molina M.T., Meléndez-Zajgla J., Carnevale A., et al. Fanconi anemia patients from an indigenous community in Mexico carry a new founder Pathogenic Variant in FANCG. Int. J. Mol. Sci. 2022;23:2334. doi: 10.3390/ijms23042334. - DOI - PMC - PubMed
-
- Kastellan S., Kalb R., Sajjad B., McReynolds L.J., Giri N., Samuel D., Milde T., Elbracht M., Holzhauer S., Niewisch M., et al. Germline biallelic BRCA2 pathogenic variants and medulloblastoma: An international cohort study. J. Hematol. Oncol. 2024;17:26. doi: 10.1186/s13045-024-01547-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

