Anti-Inflammatory and Antinociceptive Properties of the Quercetin-3-Oleate AV2, a Novel FFAR1 Partial Agonist
- PMID: 39519187
- PMCID: PMC11546106
- DOI: 10.3390/ijms252111635
Anti-Inflammatory and Antinociceptive Properties of the Quercetin-3-Oleate AV2, a Novel FFAR1 Partial Agonist
Abstract
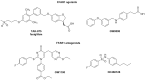
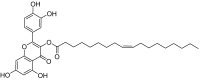
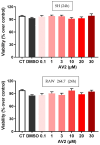
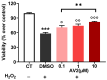
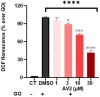
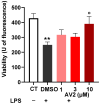
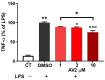
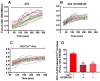
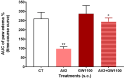
Free fatty acid receptor 1 (FFAR1) has emerged as the most targeted isoform of the free fatty acid receptors because of its involvement in the modulation of energy balance and its potential role in the control of inflammatory and pain conditions. Quercetin-3-oleate (AV2), recognized as a new FFAR1 partial agonist, was investigated for its ability to modulate inflammation and nociception. Human immortal neuroblastoma SH and the murine macrophagic RAW 264.7 cells were used to evaluate cell viability, the potential cytoprotective activity, and the anti-inflammatory properties of AV2 in vitro. Paw edema, caused by zymosan-A, and the formalin test were used to assess the in vivo anti-inflammatory and antinociceptive effects in CD-1 mice. In vitro, AV2 was devoid of cytotoxicity, significantly reduced ROS in both cell types, and protected RAW 264.7 cells from lipopolysaccharide damage by reducing tumor necrosis factor-α production. Interestingly, AV2 induced a transient elevation of intracellular calcium that was reduced in cells, pre-incubated with the FFAR1 antagonist DC260126. In vivo, AV2 reduced formalin-induced nociception and zymosan A-induced paw edema, and both effects were reversed by the FFAR1 antagonist GW1100. In conclusion, these data strongly support the AV2-mediated antioxidant, anti-inflammatory, and antinociceptive activity. AV2 represents a promising molecule for the clinical management of inflammatory-related pain conditions.
Keywords: FFAR1; inflammation; nociception; oxidative stress; quercetin-3-oleate (AV2).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hara T., Kimura I., Inoue D., Ichimura A., Hirasawa A. Free Fatty Acid Receptors and Their Role in Regulation of Energy Metabolism. Rev. Physiol. Biochem. Pharmacol. 2013;164:77–116. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

