Cellular and Molecular Pathophysiology of Gestational Diabetes
- PMID: 39519193
- PMCID: PMC11546748
- DOI: 10.3390/ijms252111641
Cellular and Molecular Pathophysiology of Gestational Diabetes
Abstract
Gestational diabetes (GD) is a metabolic disorder characterized by glucose intolerance during pregnancy, significantly impacting maternal and fetal health. Its global prevalence is approximately 14%, with risk factors including obesity, family history of diabetes, advanced maternal age, and ethnicity, which are linked to cellular and molecular disruptions in glucose regulation and insulin resistance. GD is associated with short- and long-term complications for both the mother and the newborn. For mothers, GD increases the risk of developing type 2 diabetes, cardiovascular diseases, and metabolic syndrome. In the offspring, exposure to GD in utero predisposes them to obesity, glucose intolerance, and metabolic disorders later in life. This review aims to elucidate the complex cellular and molecular mechanisms underlying GD to inform the development of effective therapeutic strategies. A systematic review was conducted using medical subject headings (MeSH) terms related to GD's cellular and molecular pathophysiology. Inclusion criteria encompassed original studies, systematic reviews, and meta-analyses focusing on GD's impact on maternal and fetal health, adhering to PRISMA guidelines. Data extraction captured study characteristics, maternal and fetal outcomes, key findings, and conclusions. GD disrupts insulin signaling pathways, leading to impaired glucose uptake and insulin resistance. Mitochondrial dysfunction reduces ATP production and increases reactive oxygen species, exacerbating oxidative stress. Hormonal influences, chronic inflammation, and dysregulation of the mammalian target of rapamycin (mTOR) pathway further impair insulin signaling. Gut microbiota alterations, gene expression, and epigenetic modifications play significant roles in GD. Ferroptosis and placental dysfunction primarily contribute to intrauterine growth restriction. Conversely, fetal macrosomia arises from maternal hyperglycemia and subsequent fetal hyperinsulinemia, resulting in excessive fetal growth. The chronic inflammatory state and oxidative stress associated with GD exacerbate these complications, creating a hostile intrauterine environment. GD's complex pathophysiology involves multiple disruptions in insulin signaling, mitochondrial function, inflammation, and oxidative stress. Effective management requires early detection, preventive strategies, and international collaboration to standardize care and improve outcomes for mothers and babies.
Keywords: chronic inflammation; gestational diabetes; insulin resistance; mitochondrial dysfunction; oxidative stress; placental dysfunction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
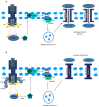



References
-
- ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., Collins B.S., Hilliard M.E., Isaacs D., Johnson E.L., et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46((Suppl. S1)):S19–S40. doi: 10.2337/dc23-S002. - DOI - PMC - PubMed
-
- Wang H., Li N., Chivese T., Werfalli M., Sun H., Yuen L., Hoegfeldt C.A., Powe C.E., Immanuel J., Karuranga S., et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022;183:109050. doi: 10.1016/j.diabres.2021.109050. - DOI - PubMed
-
- ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., Collins B.S., Hilliard M.E., Isaacs D., Johnson E.L., et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46((Suppl. S1)):S254–S266. doi: 10.2337/dc23-S015. - DOI - PMC - PubMed
-
- Tsakiridis I., Giouleka S., Mamopoulos A., Kourtis A., Athanasiadis A., Filopoulou D., Dagklis T. Diagnosis and Management of Gestational Diabetes Mellitus: An Overview of National and International Guidelines. Obstet. Gynecol. Surv. 2021;76:367–381. doi: 10.1097/OGX.0000000000000899. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

